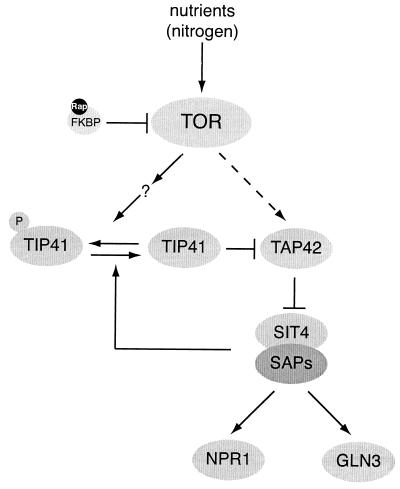
FIG. 3.
TOR controls phosphatases in S. cerevisiae. Under good nutrient (nitrogen) conditions, TOR inhibits the phosphatase SIT4 by promoting the association of SIT4 with TAP42. Two different models have been proposed for the mechanism by which TOR controls the SIT4-TAP42 complex. Jiang and Broach (81) proposed that TOR controls the interaction between SIT4 and TAP42 by phosphorylating TAP42 directly (indicated by a dashed arrow). Jacinto et al. (79) suggested that the association of SIT4 and TAP42 is controlled primarily by the TAP42 interactor TIP41. TOR may phosphorylate and inactivate TIP41 by an unknown mechanism. Dephosphorylated TIP41 positively regulates SIT4 by binding and inhibiting TAP42. The association of TIP41 with TAP42 enhances SIT4 phosphatase activity, allowing free SIT4 subunits to associate with SAPs and activate target phosphoproteins such as NPR1 and GLN3. Dephosphorylation and activation of TIP41 are mediated by SIT4, indicating that TIP41 is part of a feedback loop that amplifies SIT4 activity. Arrows indicate activation; bars indicate inhibition. Adapted from Jacinto et al. (79). Rap, rapamycin.