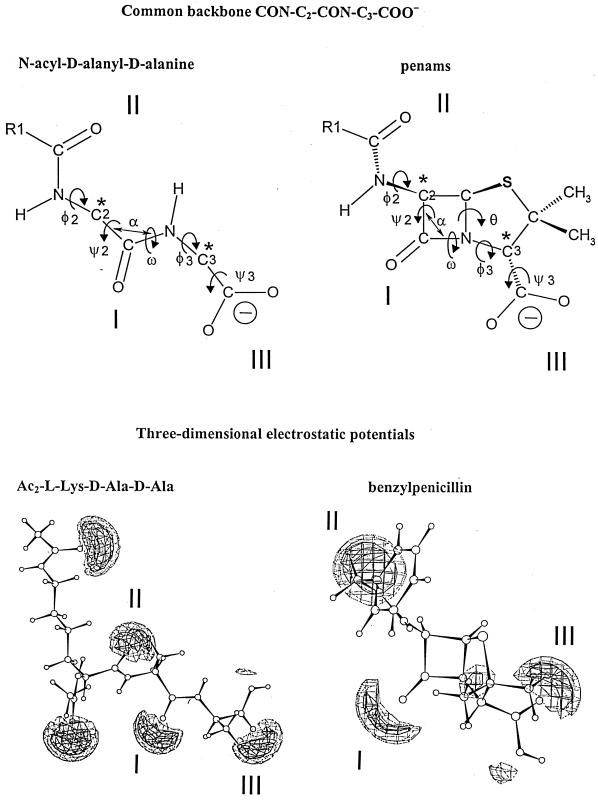
FIG. 9.
Common backbone of extended N-acyl-d-alanyl-d-alanine peptides and penams (top) and ab initio electrostatic potentials of bisacetyl-l-lysyl-d-alanyl-d-alanine and benzylpenicillin (bottom) optimized at level AM1 (the terminal carboxylates are protonated). The electrostatic negative wells I, II, and II are shown at levels of −40 kcal (solid contours) and −30 kcal (dotted contours). They are coplanar. The negative wells generated by the acetyl substituents of the α- and ɛ-amino groups of the l-lysine residue of bisacetyl-l-lysyl-d-alanyl-d-alanine are above and below the plane, respectively. The negative well of small amplitude seen between well I and well III of benzylpenicillin is due to the pair of free electrons of the nitrogen atom of the azetidinone ring. Illustration courtesy of Georges Dive, Center for Protein Engineering.