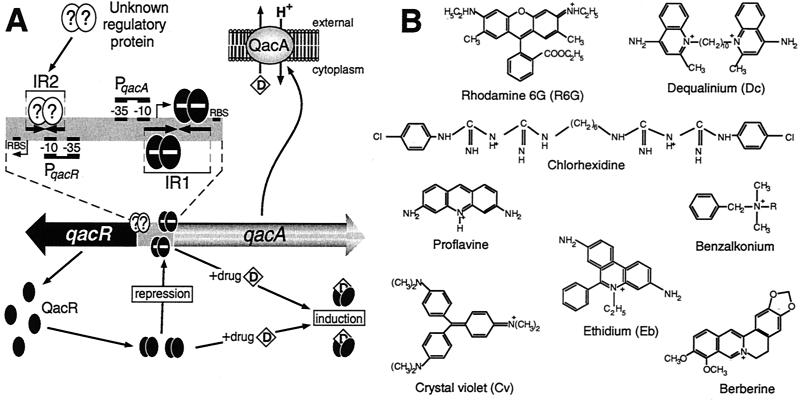
FIG. 10.
(A) Model for regulation of expression of S. aureus qacA MDR gene. QacR (black ovals) represses (−) transcription from the qacA promoter, PqacA, by binding as one dimer per IR1 half-site. Many of the lipophilic cationic drugs (D) exported from the cell by QacA in exchange for protons (H+) are also ligands of QacR. Conformational changes that occur in a QacR dimer upon drug binding result in the ligand-bound form of this protein being incapable of binding IR1, thereby mediating increases in qacA transcription in response to the presence of transporter substrates. The locations of the ribosome-binding site (RBS) and transcription start point (right-angle arrows) are indicated for both the qacA and qacR genes. Preliminary results indicate that an unknown regulatory protein (?) indirectly influences qacA expression by binding IR2, which overlaps the qacR promoter, PqacR. (B) Structurally diverse compounds that are both substrates of the QacA MDR pump and ligands of the QacR regulator. The chemical structures of representative compounds from four distinct chemical families are depicted. Dequalinium and benzalkonium (where R represents a mixture of alkyls, either C12H25, C14H29, or C16H33) are bivalent and monovalent quaternary ammonium compounds, respectively; rhodamine 6G, ethidium bromide, proflavine, and crystal violet are monovalent dyes; chlorhexidine is a bivalent guanidine; and berberine is a monovalent plant alkaloid.