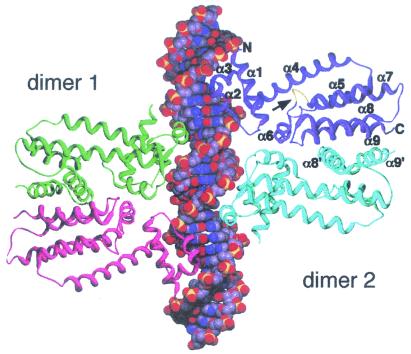
FIG. 11.
Structure of the complex formed by a pair of operator-bound QacR dimers. The two QacR dimers bound to a symmetrical version of IR1 operator DNA are depicted as ribbons, whereas the DNA is shown with the phosphate, oxygen, carbon, and nitrogen atoms colored yellow, red, grey, and blue, respectively. The DNA-reading heads from the distal subunit (purple) of dimer 2 and the proximal subunit (green) of dimer 1 contact the first major groove, whereas the second major groove of IR1 DNA is contacted by the DNA-binding domains from the other two subunits, one from each dimer. The individual α-helices (α1 to α9) of the distal subunit from dimer 2 are labeled, as are the N and C termini of that polypeptide. Additionally, the α8′ and α9′ helices from the proximal subunit of dimer 2, which form the four-helix-bundle dimerization domain with α8 and α9, are also indicated. For the distal (purple) polypeptide of dimer 2, an arrow points to the yellow region at the N terminus of α5 that undergoes a coil-to-helix transition upon ligand binding. (Reprinted with permission from reference 197; kindly provided by Maria Schumacher; ©2002, Oxford University Press.)