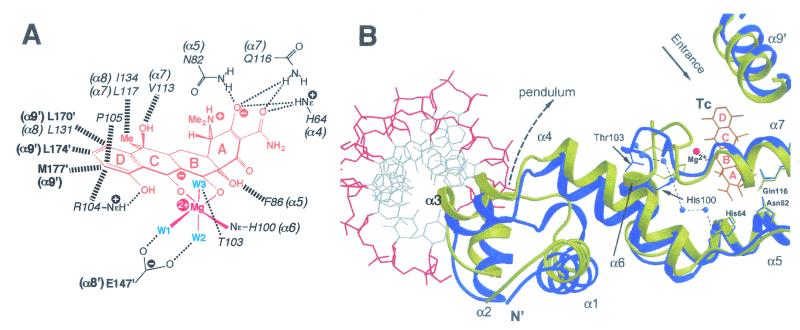
FIG. 9.
(A) Diagrammatic illustration of specific interactions between TetR(D) binding-tunnel residues and a tetracycline-Mg2+ complex. Tetracycline is colored orange, with its four rings labeled A to D and the methyl groups shown as Me. The three water molecules coordinated by the Mg2+ atom (red) are represented by W1 to W3 (blue). Residues from the TetR dimer that contribute to the binding of this tetracycline-Mg2+ complex are shown in italics for those from the first polypeptide and in bold type for those from the second polypeptide. The α-helices in which these amino acids are located are also indicated except for the Thr103, Arg104, and Pro105 residues, which form part of the interhelical loop connecting α6 to α7. Thin dotted lines indicate hydrogen bonds, and thick broken lines show hydrophobic interactions. Equivalent residues from the other binding tunnel in a TetR dimer contact a second tetracycline-Mg2+ complex; see text for other details. (B) TetR conformational changes that occur upon binding a tetracycline-Mg2+ complex. The α1 to α8 helices of one monomer and the α9′ helix from the second polypeptide in a dimer are represented in blue for DNA-bound TetR and yellow for the induced tetracycline-Mg2+-bound form. The DNA phosphate-ribose backbone is shown in red, bases in grey, tetracycline (Tc) in orange, the Mg2+ atom in red, and the chain of water molecules that constitute the water zipper in the induced conformation as blue spheres. The pendulum-like motion of α4 upon tetracycline-Mg2+ binding leads to significant displacement of the attached α1-α3 DNA-reading head, so that the α3 recognition helix can no longer contact the major groove of tet operator DNA at the same time as the α3′ recognition helix from the second polypeptide in a TetR dimer. The location of the entrance to the TetR binding tunnel is also indicated, whereas the sliding door motion of α9′ that closes this entrance in the induced form is also apparent. See text for other details. (Panel A reprinted with permission from reference 60; ©1994, American Association for the Advancement of Science. Panel B reprinted with permission from reference 153; kindly provided by Winfried Hinrichs.)