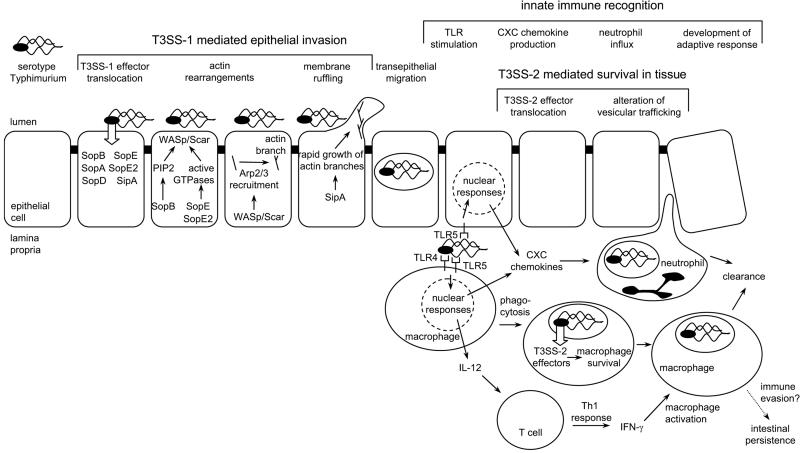
FIG. 1.
Pathogenesis of serotype Typhimurium-induced gastroenteritis in humans. Serotype Typhimurium T3SS-1 injects effector proteins (SipA, SopA, SopB, SopD, SopE, and SopE2) into epithelial cells, which mediate bacterial invasion. Phosphatidylinositol phosphates (PIP2) produced by SopB and Rho family GTPases activated by SopE/SopE2 act in concert to activate WASp/Scar proteins, which in turn recruit the Arp2/3 complex to form new branches on actin filaments. SipA accelerates the growth of new branches, resulting in the formation of membrane ruffles and bacterial internalization. Once serotype Typhimurium has crossed the epithelial barrier, its presence in the lamina propria is detected by pathogen recognition receptors (TLR4 and TLR5) and the organism is internalized by phagocytes. TLR signaling in epithelial cells and mononuclear cells (macrophages and dendritic cells) results in the release of neutrophil chemoattractants (CXC chemokines) which trigger the massive neutrophil influx that is the hallmark of inflammatory diarrhea. Serotype Typhimurium is able to survive within macrophages by using its T3SS-2 to translocate into the host cell cytosol effectors that enable the pathogen to evade phagocyte killing mechanisms by altering vesicular trafficking. TLR signaling also triggers the release of IL-12 from mononuclear cells, thereby initiating the development of a protective Th1 response. With the development of adaptive immune responses (e.g., IFN-γ activation of macrophages), host phagocytes are able to clear the infection and symptoms subside. Some bacteria escape clearance and persist for 1 or 2 months in an intestinal niche. Recently identified mechanisms for immune evasion by serotype Typhimurium could be important during this phase of intestinal persistence (4, 10, 53).