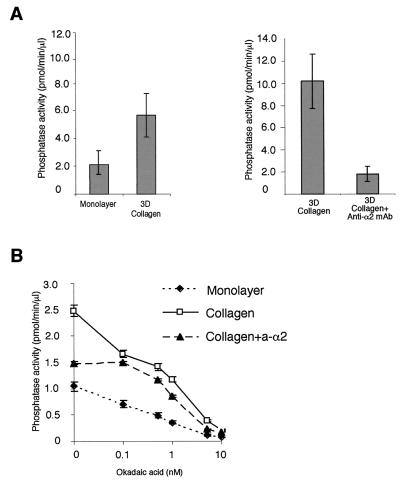
FIG. 4.
Serine/threonine phosphatase PP2A is activated inside 3D collagen gel. Fibroblasts were serum starved and then harvested from the cell culture dish (monolayer) or seeded inside collagen gel for 1.5 h. Serum-starved fibroblasts were detached and placed inside collagen in the presence or absence of either an anti-α2 function-blocking MAb (collagen + anti-α2; 2.5 μg/ml) or a nonfunctional anti-α1 MAb (2.5 μg/ml; not shown). Thereafter, cells were lysed and equal amounts of protein were assayed for PP1-PP2A activity using 32P-labeled glycogen phosphorylase as a substrate. Phosphatase activity of cells seeded inside collagen versus that of cells cultured in a monolayer and the effect of anti-α2 MAb are shown (A). To distinguish between PP1 and PP2A type activity, the effect of okadaic acid (causes half-maximal inhibition of PP2A at 0.1 nM and has no effect on PP1 below 5 nM) was tested by an in vitro phosphatase reaction (B). Phosphatase activity relative to cell lysate protein content (mean ± SEM; n = 3) is shown.