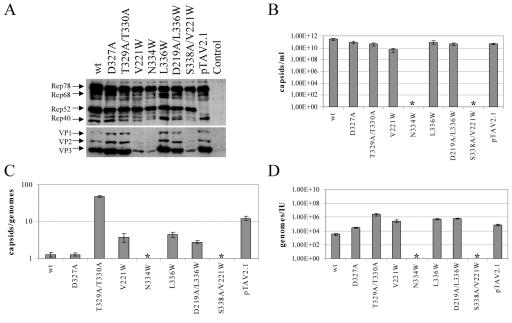
FIG. 2.
Characterization of new AAV2 pore mutants. Viral supernatants were generated from 293T cells transfected with AAV2 wt or mutated genomic plasmids or a small Rep-deficient mutant (pTAV2.1) (25) or Bluescript II as controls. Supernatants were assayed for viral protein expression (Western blot analysis using monoclonal anti-Rep [303.9] or anti-VP [B1] antibodies) (A) or ELISA-based AAV2 capsid titer (B). Influence of the mutations on genome packaging and infectivity was quantified by calculating the ratios of capsids to genomes (C) and genomes to infectious units (IU) (D), respectively. Means ± standard deviations from at least four independent experiments are shown; an asterisk indicates mutants for which capsids could not be detected in the ELISA (detection limit, 5 × 108 capsids/ml).