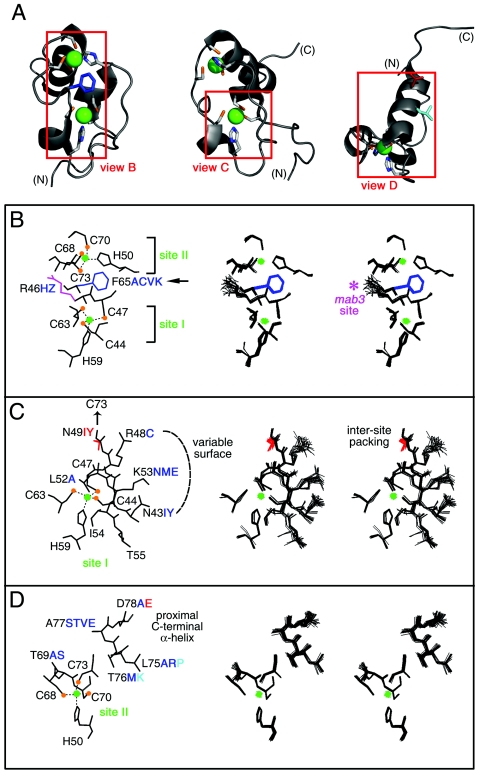
FIG. 11.
Structural environments of mutation sites. (A) Ribbon models of DSX DM domain. Regions boxed in red are shown in panels B, C, and D, respectively. Zinc ions are shown in bright green, and Zn coordinating cysteines are tipped in orange. The F65 side chain is shown in dark blue (B), that for D78 in red (D), and that for L75 in light blue (D). (B through D) Local environments of selected side chains. In each panel, one representative model is labeled at left; the corresponding DG/SA ensemble is shown in stereo at right. Thiolate-Zn bonds in each panel are shown as dotted lines. Color schemes follow that of panel A. Mutations that impair reporter gene expression are shown in red. Mutations that have no or partial effects are shown in blue and aqua. (B) Zn coordination sites. Residues C44, C47, H59, and C63 comprise site I; residues H50, C68, C70, and C73 comprise site II. Conserved residues R46 and F65 are also shown. Mutation of R46 (side chain shown in magenta) to W in the homologous sequence of mab-3 in C. elegans causes intersexual development of chromosomal male. Residue F65 functions as a bridge between the two Zn-binding sites, participating in an aromatic-aromatic interaction with H50 and additional side chain interactions with several residues near site I (not shown). (C) Packing of ordered residues near site I allows for the formation of a well-defined polar or basic surface. The buried side chain of N49 interacts with C73, promoting the formation of Zn-binding site II. The side chains of residue N43, R48, and K53 form a hydrophilic polar surface. Ordered residues L52, I54, and T55 underlie this surface. (D) Packing of ordered residues near site II. The side chains of α-helical residues in the C-terminal region are also shown. These presumably nucleate helix propagation on DNA binding.