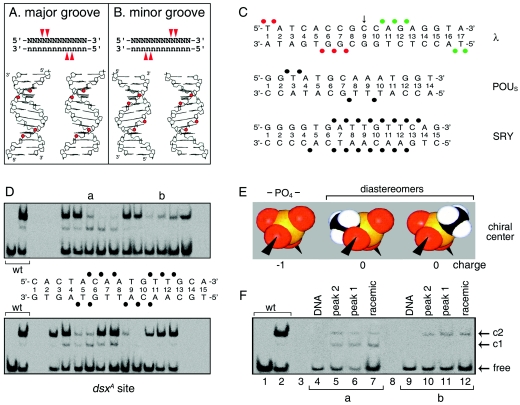
FIG. 7.
Methylphosphonate footprint of DSX DM domain. (A and B) Classical backbone footprints of major- and minor-groove DNA-binding proteins. Binding of a small globular domain to one face of B DNA yields a staggered pattern of phosphate contacts (red arrowheads) whose orientation depends on which groove is occupied (14, 68). (C) Patterns of protein contacts to DNA phosphodiester groups; sites of interference are depicted as filled circles. Major-groove patterns are observed in λ repressor (top) (36, 37) and human Oct-2 POUS domains (middle) (10). The SRY HMG box (bottom) exhibits a nonclassical pattern due to DNA bending and unwinding (51, 58). Contacts by symmetry-related protomers in λ complex are shown in red and green. (D) GMSA methylphosphonate interference. Sites of interference are indicated by filled circles. Phosphodiester positions across 15-bp DNA site in complementary strands are designated by a common number based on nucleoside position in the upper strand. Base pairs are numbered 1 to 15 from left. (E and F) Test for stereospecific interference following HPLC resolution of Sp and Rp diastereomers. (E) Corey-Pauling-Koltun (CPK) models of negatively charged phosphodiester linkage (PO4−, left) and neutral isomers (center and right). Phosphorus is shown in orange, oxygen in red, and the methyl group in black and white. (F) Representative gels showing absence of stereospecific interference at two sites of partial interference (a and b).