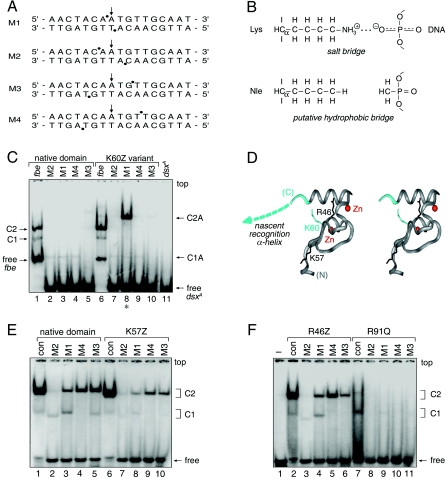
FIG. 9.
Chemogenetic analysis of putative protein-DNA contact. (A) DNA probes defined by the DSX DM domain footprint. Sites of interference (filled circles) are nearly symmetric about the central base pair (arrow). (B) Chemogenetic strategy envisages substitution of salt bridge at protein-DNA interface (upper panel) by hydrophobic bridge (lower panel). (C) GMSA autoradiogram results for specific binding of native protein (lanes 1 to 5) or K60Z variant (lanes 6 to 10) to 33P-labeled DNA sites. Native and variant protein concentrations were 24 nM and 500 nM, respectively. Control lanes 1 and 6 demonstrate binding to unmodified dsxA site within 29-bp fat body enhancer (fbe) duplex. Native 1:1 and 2:1 complexes are respectively labeled C1 and C2 (fbe) and C1A and C2A (M1 to M4). Binding of native protein to modified dsxA probes M1 to M4 (lanes 2 to 5) yields only a weak 1:1 complex. Whereas binding of K60Z variant to probes M2 to M4 is likewise perturbed, formation of a 2:1 complex is rescued by modification M1 (lane 8, asterisk). Note accidental similarity of motilities between free fbe probe in lanes 1 and 6 (29 bp) and the C1 complex containing dsxA (15 bp; lanes 2 to 5). (D) Ribbon model (stereo pair) of DSX Zn module (DSX residues 35 to 78; gray) and tail (dashed line; azure). Sites of norleucine substitution and zinc ions (red spheres) are shown. (E and F) GMSA screening of DSX analogs against methylphosphonate DNA probes M1 to M4. No specific pattern of second-site compensation is observed for native domain and K57Z (E) or R46Z and R91Q (F) variant proteins. The native domain concentration was 120 nM, whereas the concentrations of the DSX analogs were in each case 520 nM. The control lanes (con, lane 1 in panel E and lane 2 in panel F) employ a 15-bp dsxA duplex site containing a methylphosphonate modification outside of the footprint (i.e., at a noninterfering site). Respective percentages of DNA probe shifted to the C1 and C2 forms for the native domain were as follows: control, 2% and 94%; M2, 5% and 3%; M1, 11% and 23%; M4, nondetectable and 43%; and M3, 5% and 50%. Respective percentages for the K57Z variant were as follows: control, 1% and 91%; M2, <1% and 3%; M1, 2% and 9%; M4, nondetectable and 18%; and M3, nondetectable and 17%. Respective percentages for the R46Z variant were as follows: control, 3% and 78%; M2, 1% and nondetectable; M1, 7% and 18%; M4, nondetectable and 34%; and M3, nondetectable and 14%. Respective percentages for the R91Q variant were as follows: control, 10% and nondetectable; M2, both nondetectable; M1, 1% and nondetectable; M4, both nondetectable; and M3, <0.5% and nondetectable.