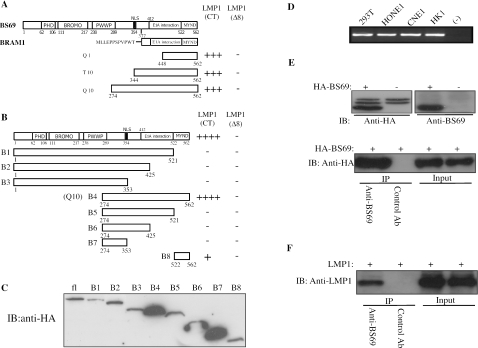
FIG. 2.
BS69 interacts with LMP1 in yeast and mammalian cells. (A and B) Schematic representation of BS69, BRAM1, and various truncated fragments of BS69. The numbers underneath each bar indicate the positions of amino acids in BS69. NLS, nuclear localization sequence; CT, carboxyl terminus. The interaction of full-length BS69 and its truncated derivatives with either LMP1(CT) or LMP1(Δ8) was analyzed by yeast two-hybrid assays, and the results are summarized next to each construct on the right. Q1, T10, and Q10 were three BS69 fragments isolated from our yeast two-hybrid screen. ++++ and +++ represent the appearance of yeast colonies in 3 and 5 days, respectively, after being streaked on QDO plates. ++ and + indicate the appearance of yeast colonies in 3 and 5 days, respectively, on TDO plates (these clones did not grow on QDO plates). −, no growth on either QDO or TDO plates. (C) The expression levels of different BS69 fragments in yeast were detected by immunoblotting. fl, full-length. (D) Total RNA was extracted from 293T, HONE1, CNE1, and HK1 cells; an equal amount of RNA was subjected to RT-PCR analysis. (−), negative control without the reverse transcriptase. (E) 293T cells were either mock transfected or transfected with HA-BS69. (Top) WCEs were subjected to direct immunoblot analysis. (Bottom) WCEs were immunoprecipitated separately with either the anti-BS69 antibody or a control antibody (Ab), followed by immunoblotting with an anti-HA antibody. (F) 293T cells were transfected with LMP1. Cells were cross-linked with dithiobis(succinimidylpropionate) for 5 min before harvest. The lysates were immunoprecipitated with either the anti-BS69 antibody or a control antibody, followed by immunoblotting with the anti-LMP1 antibody.