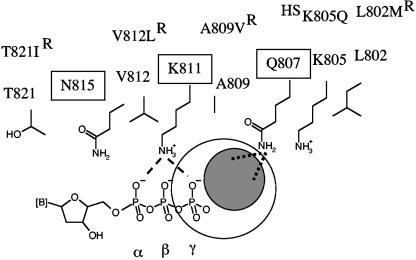
FIG. 6.
Possible interactions between helix P and foscarnet. This simplified model shows side chains of important residues in helix P that are either highly conserved (boxed) among α-DNA polymerases or associated with changes in susceptibility to the drug (R, resistant; HS, hypersusceptible). Helix P of UL54 is shown here in an orientation to the bound nucleotide similar to that shown for helix P of gp43 in Fig. 1C. The structural data suggest that positions 805 and 802 are likely to be too far away to provide direct contacts with the drug. Our data suggest that foscarnet may bind in close proximity to Q807. The gray circle indicates the possible location of the bound drug relative to the nucleotide. The orientation of the bound foscarnet cannot be predicted on the basis of the data presented in this study. Dotted lines illustrate possible interactions between the negatively charged foscarnet and the side chain of Q807. There might be a partial overlap with the position of the γ-phosphate (large open circle), which would help to explain previous data that suggested a competitive mode of inhibition of pyrophosphorolysis. Dashed lines illustrate contacts between the structural equivalent of K811 (K560) in gp43 and the α- and γ-phosphates of the bound dNTP. Possible interaction between K811 and foscarnet cannot be confirmed, because mutations at this position were shown to affect dNTP binding at the same time.