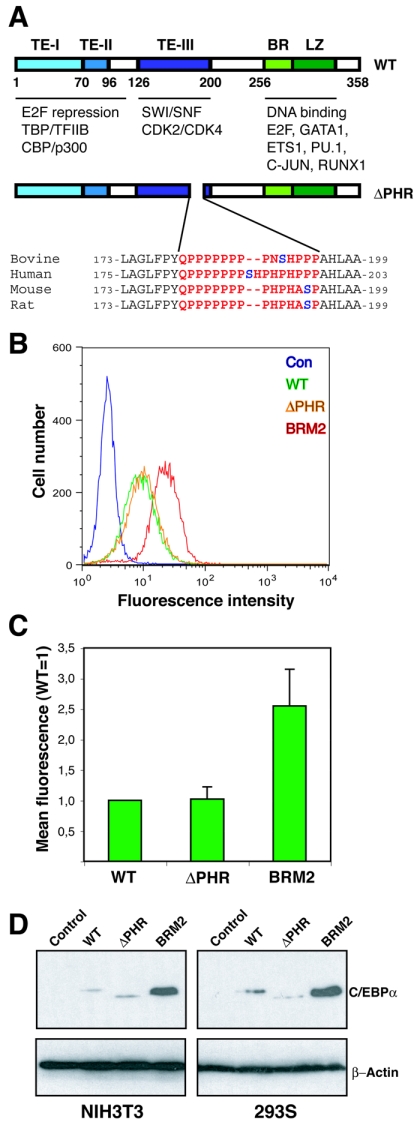
FIG. 1.
In vitro growth repression of continuously expressed C/EBPαΔPHR variant proteins. (A) Schematic representation of the C/EBPα protein showing the three transactivation elements (20), the DNA binding basic region (BR), and the leuzine zipper dimerization domain (LZ). Protein interaction partners are indicated below the protein. The ΔPHR variants (rat numbering) are also shown. The PHR sequences from four mammals are highlighted in red, and the putatively phosphorylated serine is shown in blue. (B) The eGFP fluorescence of NIH 3T3 cultures transduced with retroviruses derived from Phoenix-E cultures transfected with pBabePuro (Con), pBabePuro-C/EBPα-eGFP (WT), pBabePuro-C/EBPαΔPHR-eGFP (ΔPHR), or pBabePuro-C/EBPα-BRM2-eGFP (BRM2) was determined by flow cytometry after 14 days of culturing at subconfluency (approximately 15 cell divisions). (C) The mean eGFP fluorescence (autofluorescence subtracted from NIH 3T3 cells) was plotted from three independent experiments. Errors bars indicate standard deviations. (D) C/EBPα levels in NIH 3T3 (retrovirally transduced with pBabePuro derivatives) or HEK293S cultures (transfected with pcDNA3 derivatives) expressing C/EBPα (WT), C/EBPαΔPHR (ΔPHR), or C/EBPαBRM2 (BRM2). Controls (Con) indicate pBabePuro (NIH 3T3) or pcDNA3 (HEK293). Cells were kept subconfluent under selective conditions for 3 weeks, and lysates were prepared and subjected to Western blotting using a C/EBPα-specific antibody. A β-actin antibody was used to control for even loading. TE-I, TE-II, and TE-III, transactivation elements I, II, and III, respectively.