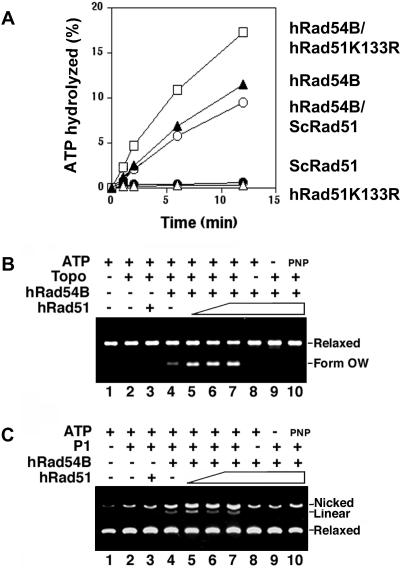
FIG. 3.
Human Rad54B and human Rad51 functionally interact. (A) Human Rad51 stimulates the ATPase activity of hRad54B. Human Rad54B (120 nM) was incubated with [γ-32P]ATP and various forms of Rad51, and the percentage of ATP hydrolyzed was determined. A significant stimulation of hRad54B ATPase activity was detected with hRad51 K133R (300 nM), a mutant variant of hRad51 that can bind but not hydrolyze ATP. The effect is species specific, because Rad51 from S. cerevisiae (ScRad51; 300 nM) did not affect the ATPase activity of hRad54B. (B) Human Rad51 stimulates hRad54B in the translocase assay. The formation of form OW DNA was determined in the presence of topoisomerase I (Topo), hRad54B (300 nM), and hRad51 (0, 200, 300, and 400 nM, respectively, in lanes 4 to 7). In control reactions, hRad51 (400 nM) and DNA were incubated with topoisomerase and ATP but no hRad54B (lane 3), with hRad54B and ATP but no topoisomerase (lane 8), with hRad54B and topoisomerase but no ATP (lane 9), or with hRad54B, topoisomerase, and AMP-PNP (lane 10). DNA alone (lane 1) and DNA incubated with topoisomerase (lane 2), both in the presence of ATP, were also analyzed. Form OW is the positively supercoiled DNA species generated. (C) Human Rad51 stimulates hRad54B in the P1 nuclease assay. The ability of hRad54B to open up the DNA double helix is enhanced by the presence of hRad51. hRad54B (100 nM) was incubated with topologically relaxed DNA, ATP, P1 nuclease, and hRad51 (100, 200, and 300 nM in lanes 5 to 7, respectively) or without hRad51 (lane 4). In control reactions, hRad51 (300 nM) and DNA were incubated with P1 and ATP but no hRad54B (lane 3), with hRad54B and ATP but no P1 (lane 8), with hRad54B and P1 but no ATP (lane 9), or with hRad54B, P1, and AMP-PNP (lane 10). DNA alone (lane 1) and DNA incubated with P1 (lane 2), both in the presence of ATP, were also analyzed.