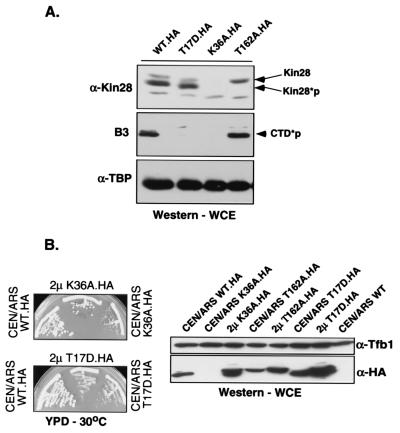
FIG. 1.
In vivo analysis of Kin28 mutants. (A) Protein levels. The indicated HA-tagged Kin28 mutants were used to replace the product of the wild-type (WT) gene in yeast by plasmid shuffling. Total proteins extracted from exponentially growing cultures at 30°C in yeast extract-peptone-dextrose (YPD) medium were resolved by SDS-PAGE, and levels of Kin28 (anti-Kin28 [α-Kin28], rabbit polyclonal antibody), phosphorylated Rpb1 CTD (CTD*p; B3 monoclonal antibody), and TATA-binding protein (TBP) (anti-TBP [α-TBP], rabbit polyclonal antibody) were assayed by immunoblotting. Low levels of the K36A protein are detectable following prolonged exposure (not shown). WCE, whole-cell extract. (B) Overexpression of Kin28(K36A) partially suppresses the slow-growth phenotype the mutant produces. Plasmid shuffling was carried out using low-copy-number (CEN/ARS) or high-copy-number (2μm) plasmids carrying the indicated mutant kin28 genes. Strains were streaked on YPD plates and incubated at 30°C for 3 days (left). The mutant strains were also assayed by immunoblotting (right) for Kin28 protein levels using the 12CA5 monoclonal antibody, which recognizes the HA tag (α-HA). A polyclonal antibody against Tfb1 (α-Tfb1) was used as a control for core TFIIH levels.