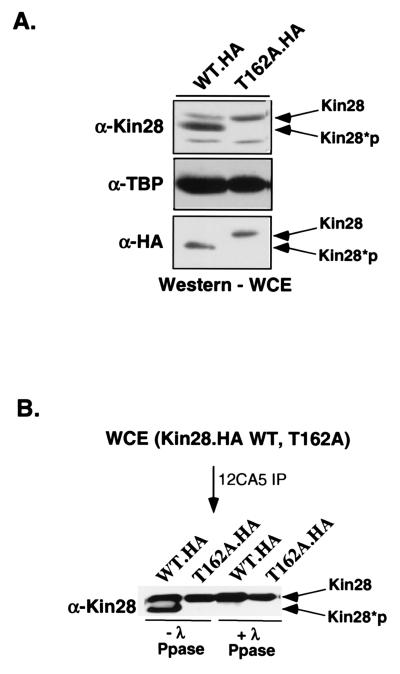
FIG. 3.
Kin28 is phosphorylated on T162 within the T loop. (A) Immunoblot analysis of wild-type (WT) Kin28 whole-cell extracts (WCE) shows that the protein migrates as a doublet corresponding to the phosphorylated and unphosphorylated species. The T162A mutant protein migrates as a single band. Kin28*p, phosphorylated Kin28; TBP, TATA-binding protein. (B) HA-tagged Kin28 was immunoprecipitated (IP) via the HA tag using the 12CA5 monoclonal antibody and treated with λ-phosphatase (Ppase). The faster-migrating band disappears, indicating that the mobility shift is phosphorylation dependent. The HA-tagged Kin28(T162A) [Kin28.HA(T162A)] mutant was used as a gel mobility standard for the unphosphorylated form.