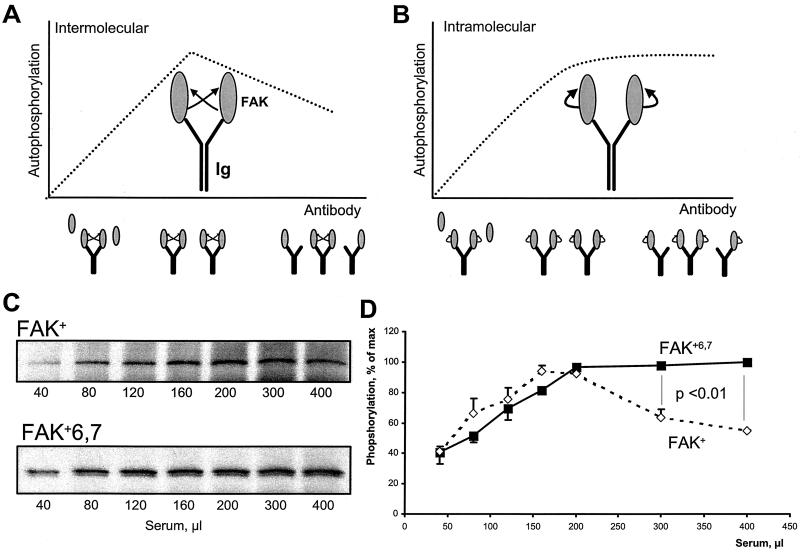
FIG. 6.
Different mechanisms of autophosphorylation of FAK+ and FAK+6,7. (A and B) Principle of the CITIK assay. Equal amounts of lysates from COS-7 cells transfected with FAK are immunoprecipitated in the presence of increasing amounts of antiserum and constant amounts of protein A-Sepharose. Autophosphorylation assays are carried out in the immune precipitates, and the results are expected to be affected by the reaction mechanism as follows. For small amounts of antiserum, the quantity of specific antibodies against FAK is limiting, and all the available binding sites are expected to be occupied, as schematically indicated by two molecules of FAK per immunoglobulin (Ig). In these conditions, autophosphorylation increases with the amount of antiserum, reflecting the increasing amount of immunoprecipitated FAK, regardless of the cis or trans mechanism (ascending part of the curve). When the quantity of serum is further increased, the number of FAK-specific binding sites becomes greater than the number of FAK molecules, and an increasing proportion of immunoglobulin is expected to bind only one molecule of FAK or none. In these conditions, the trans and cis mechanisms of autophosphorylation can be clearly distinguished: intermolecular autophosphorylation will diminish with increasing the amount of serum, since the probability that two FAK molecules will be bound to the same immunoglobulin will decrease (A); intramolecular autophosphorylation is independent of immunoglobulin G-mediated interactions and will reach a plateau corresponding to the total amount of FAK (B). CITIK assays were carried out for FAK+ and FAK+6,7 as described in the text. Reaction products were separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by immunoblotting (C), and incorporated 32P was quantified with an Instant Imager (D). The total amount of immunoprecipitated FAK, estimated by immunoblotting with FAK antibodies, was maximal with approximately 200 μl of antiserum and remained constant with higher amounts of antiserum (C). The amount of 32P incorporated was plotted as a function of the amount of antiserum for the two FAK isoforms (D). Results are expressed as percent of maximum to correct for the higher activity of FAK+6,7 compared to FAK+. Results are means ± standard error of the mean of three independent experiments. Statistical analysis was done with two-way analysis of variance. The results show a significant difference between FAK+6,7 and FAK+ for the relevant portion of the curve, supporting the hypothesis that phosphorylation is intramolecular in the case of FAK+6,7 and intermolecular in the case of FAK+.