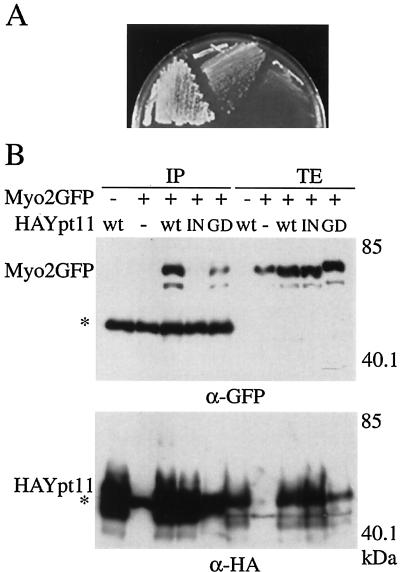
FIG. 1.
Functional and physical interactions between Myo2p and Ypt11p. (A) Suppression of myo2-66 with a high dose of YPT11. myo2-66 cells (strain YMK021) with MYO2 on a low-copy-number plasmid (left), with a high-copy-number plasmid carrying YPT11(pK008, middle), or with a control vector (pYO326, right) were streaked on an SC plate lacking uracil and incubated at 30°C for 3 days. (B) Complex formation between Ypt11p and the C-terminal tail domain of Myo2p. ypt11Δ cells (strain yTO001), carrying pGAL1-Myo2 for producing the GFP-tagged C-terminal tail domain of Myo2p (Myo2GFP +), YEpGAL1 as a control vector (Myo2GFP −) with the indicated plasmid for HA-tagged Ypt11p (wt, HA-tagged Ypt11p from p901EW-HA-YPT11; IN, HA-tagged Ypt111144Np from p901EW-HA-YPT111144N; GD, HA-tagged Ypt11G40Dp from p901EW-HA-YPT11G40D), or control vector p901EW (−), plasmid YEplac112 (12) with an insert for the HA-tag, were disrupted, and the resulting cell extracts were immunoprecipitated with anti-HA antibodies. The total cell lysate (TE; 1 μg of protein from the cell lysate) and immunoprecipitate (IP; precipitate from 1.6 mg of protein from the cell lysate) were analyzed by Western blotting using anti-GFP antibodies (top) and anti-HA antibodies (bottom). The migration positions of GFP-tagged Myo2p and HA-tagged Ypt11p are indicated by Myo2p-GFP and HA-Ypt11p, respectively. Asterisks indicate the migration positions of IgG. Numbers at the right indicate the molecular mass of the protein marker in kilodaltons. About 0.06% of the Myo2p tail in the lysate was coimmunoprecipitated with the wild-type HA-Ypt11p, judging from the figure. Ypt11p migrated more slowly in the sodium dodecyl sulfate gel than expected from its calculated molecular mass. Bacterially produced Ypt11p also migrated as slowly as Ypt11p in the lysate, suggesting that the slow migration of Ypt11p is due to its protein nature rather than a consequence of posttranslational modification.