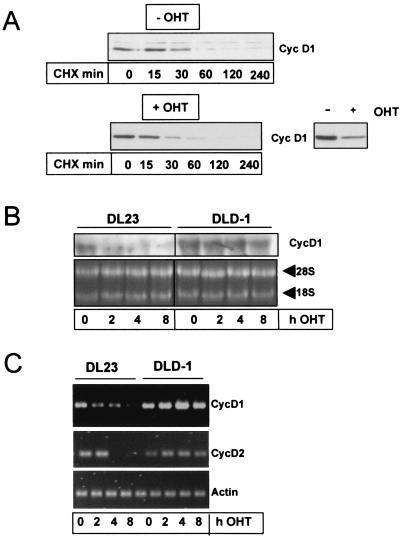
FIG. 5.
Activation of FoxO3a represses cyclin D transcription but does not affect protein stability of cyclin D1. (A) DL23 cells were either pretreated with 100 nM 4-OHT (+OHT, lower panel) or left untreated (−OHT, upper panel). After 6 h, cells were incubated with 10 μg of cycloheximide/ml (CHX) to prevent de novo protein synthesis. At the indicated time points after CHX addition, cells were lysed and the effect of 4-OHT-mediated FoxO3a activation on the stability of cyclin D1 protein was assessed by Western blotting. The inset demonstrates the effectiveness of 4-OHT-mediated FoxO3a activation in this experiment, as 4-OHT treatment alone resulted in reduced cyclin D1 protein levels compared to those of uninduced cells (lower right panel). To compare protein degradation in the presence or absence of 4-OHT, a longer exposure of the blot from the 4-OHT-stimulated cells is shown. (B and C) DL23 cells or DLD-1 cells were stimulated with 100 nM 4-OHT for the indicated times, and total RNA was isolated. The samples were then analyzed for mRNA levels of cyclin D1, cyclin D2, and actin (control) by semiquantitative RT-PCR (C) or for cyclin D1 mRNA by Northern blotting (B). As a loading control for panel B, an ethidium bromide staining of the 28S and 18S ribosomal RNAs is shown. RT-PCRs were shown to be in a linear range by performing parallel control PCRs with increasing template cDNA concentrations (not shown).