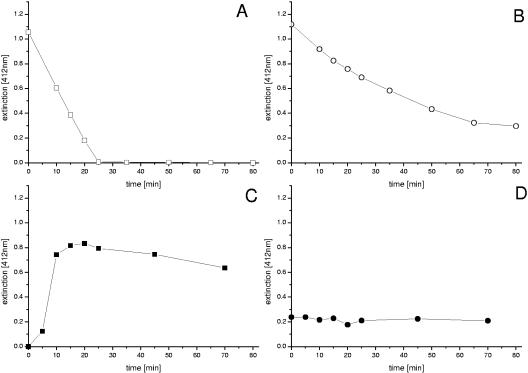
FIG. 4.
Characterization of cystathionine-γ-synthase (MetB) and cystathionine-β-lyase (MetC) from C. glutamicum after overexpression as His-tagged proteins in E. coli and subsequent purification. The conversion of O-acetylhomoserine and l-cysteine (A) and O-acetylhomoserine and l-homocysteine (B) by MetB and the cleavage of cystathionine (C) and l-homolanthionine (D) by MetC were monitored by photometric measurement of free SH groups via Ellman's reagent at 412 nm. l-Homolanthionine was added from the assay mixture of the MetB assay after removal of the MetB enzyme by three cycles of filtration (molecular weight cutoff, 20,000) (Centrisart; Sartorius, Göttingen, Germany). This solution contained primarily homolanthionine and to some extent remaining homocysteine and O-acetylhomoserine, which explains the initial levels of free SH groups in the assay (D).