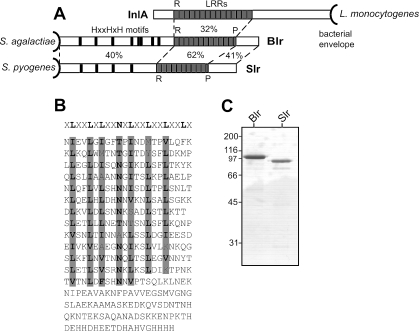
FIG. 1.
Analysis of the Blr protein of S. agalactiae. (A) The Blr protein exhibits extensive residue identity to Slr of S. pyogenes (46), and both of these predicted lipoproteins are related to L. monocytogenes InlA, which is attached to the wall via an LPXTG motif. Amino acid residue identities of different regions are indicated in percents between the proteins. In all three proteins, the regions with LRRs are probably located distally to the bacterial surface. There are 12.5 LRRs in Blr, 10.5 LRRs in Slr, and 15 LRRs in InlA. The positions of histidine triad motifs (HXXHXH) in the N-terminal part of Blr and Slr are indicated. R, repeat; P, partial repeat. (B) Amino acid sequence of the C-terminal part of the Blr protein, including the LRR domain. The consensus sequence of LRRs in L. monocytogenes InlA is indicated in bold at the top, and the corresponding residues in the LRRs of Blr are highlighted in gray. In Blr, each LRR has a length of 22 residues, with the exception of the seventh repeat, which is one residue shorter. (C) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified recombinant Blr and Slr. Molecular weight markers (in thousands) are noted at the left of the gel.