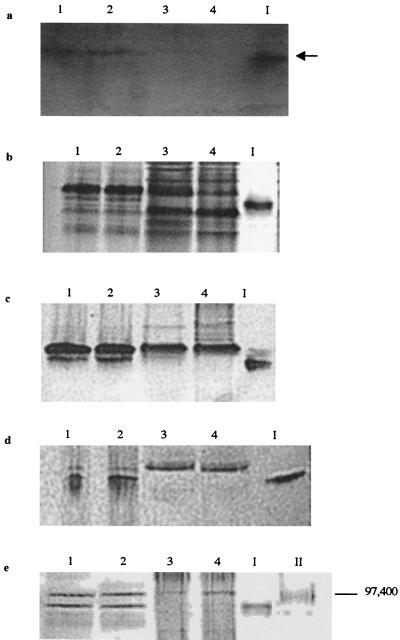
FIG. 7.
Separation of peripheral proteins of insertional mutant E912 via native PAGE with ferrozine staining (5 min) (a), Coomassie blue staining (b), and heme staining (c) and via SDS-PAGE with heme staining (d) and Coomassie blue staining (e). Lanes contain peripheral proteins washed from whole cells of 200R(pBBR1MCS) (lanes 1), complemented transconjugate B31(pB4-2S-C) (lanes 2), Fer− transconjugate B31(pBBR1MCS) (lanes 3), and insertional mutant E912 (lanes 4). The 91-kDa heme-containing band displaying Fe(III) reduction activity was excised from an unstained native gel and is included as a marker in lane I of all gels. Lanes II contain SDS-PAGE molecular mass standards Bio-Rad) E. coli β-galactosidase (116,250 Da) and rabbit muscle phosphorylase b (97,400 Da). The arrow indicates a band displaying Fe(III) reduction activity (the black color on the image corresponds to magenta-colored Fe2+-ferrozine complex on the actual gel).