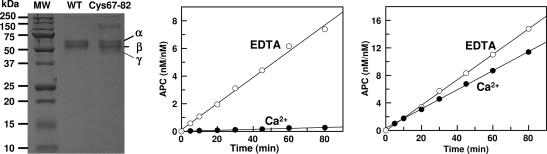
Fig. 1.
SDS/PAGE analysis of wild-type and mutant GD-PC and the time course of the initial rate of zymogen activation by thrombin in the presence of EDTA and Ca2+. (Left) One microgram of each protein was applied to 10% SDS/PAGE under nonreducing conditions. The subforms α (≈55 kDa), β (≈52 kDa), and γ (≈50 kDa) are glycosylation variants of protein C (10). (Center) The time course of activation of wild-type GD-PC (1 μM) by thrombin (10–50 nM) was monitored in TBS/EDTA (○) or TBS/Ca2+ (•) as described in Methods. The activation rates were as follows: 0.003 nM/min in Ca2+ and 0.09 nM/min in EDTA. (Right) The same as above except that the activation of Cys-67–82 protein C by thrombin (10 nM) was monitored in either EDTA (○) or Ca2+ (•). The activation rates were as follows 0.13 nM/min in Ca2+ and 0.18 nM/min in EDTA.