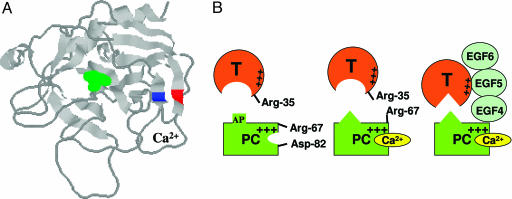
Fig. 6.
Crystal structure of the catalytic domain of APC and the proposed model of protein C activation by thrombin in the absence and presence of Ca2+ and TM. (A) The three-dimensional positions of Arg-67 (blue) and Asp-82 (red) on two antiparallel β structures of APC are shown. The catalytic residue Ser-195 is shown in green. The coordinates (Protein Data Bank entry 1AUT) were used to prepare the figure (8). (B Left) In the proposed model, Arg-67 and Asp-82 of protein C (PC) are involved in electrostatic interactions in the absence of Ca2+. (B Center) The binding of Ca2+ to protein C disrupts this interaction, relocating Arg-67 to an inhibitory position for interaction with thrombin in the absence of TM. The metal ion also changes the conformation of the activation peptide (AP) of protein C. (B Right) TM binds to exosite-1 of thrombin via its EGF5 domain and to the basic exosite of the Ca2+-stabilized protein C (including Arg-67) via its EGF4 domain, thereby altering the conformation of the active-site/extended binding pocket of thrombin and facilitating the docking of the activation peptide of protein C in the catalytic groove of thrombin (see text for further discussion).