Abstract
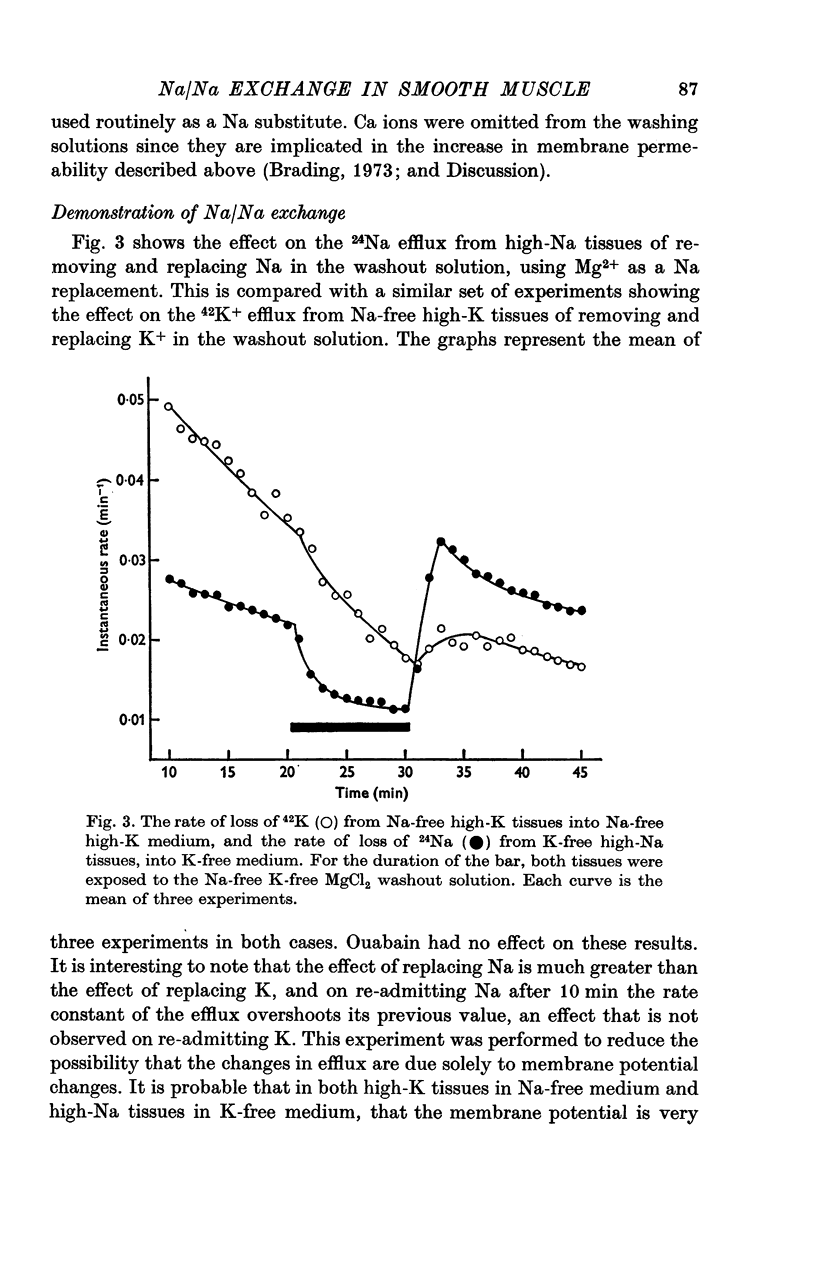
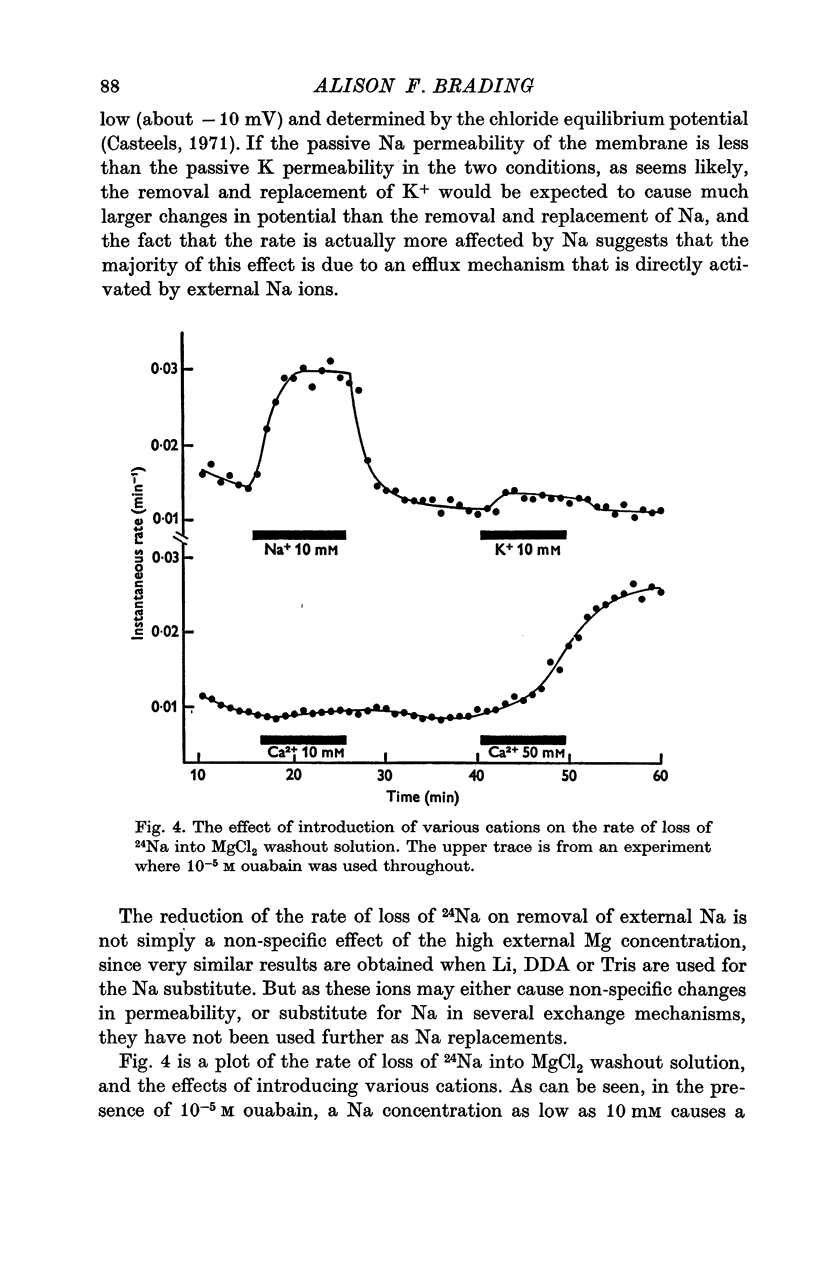
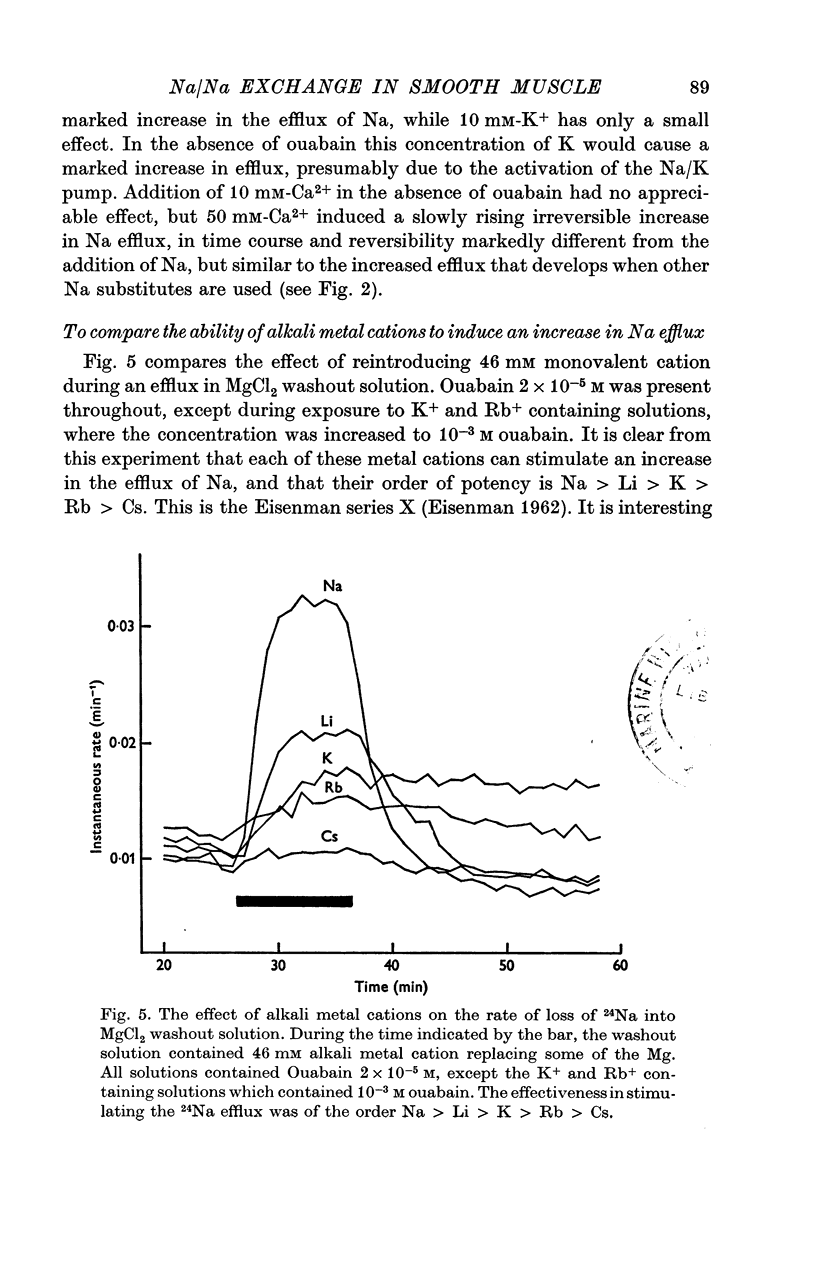
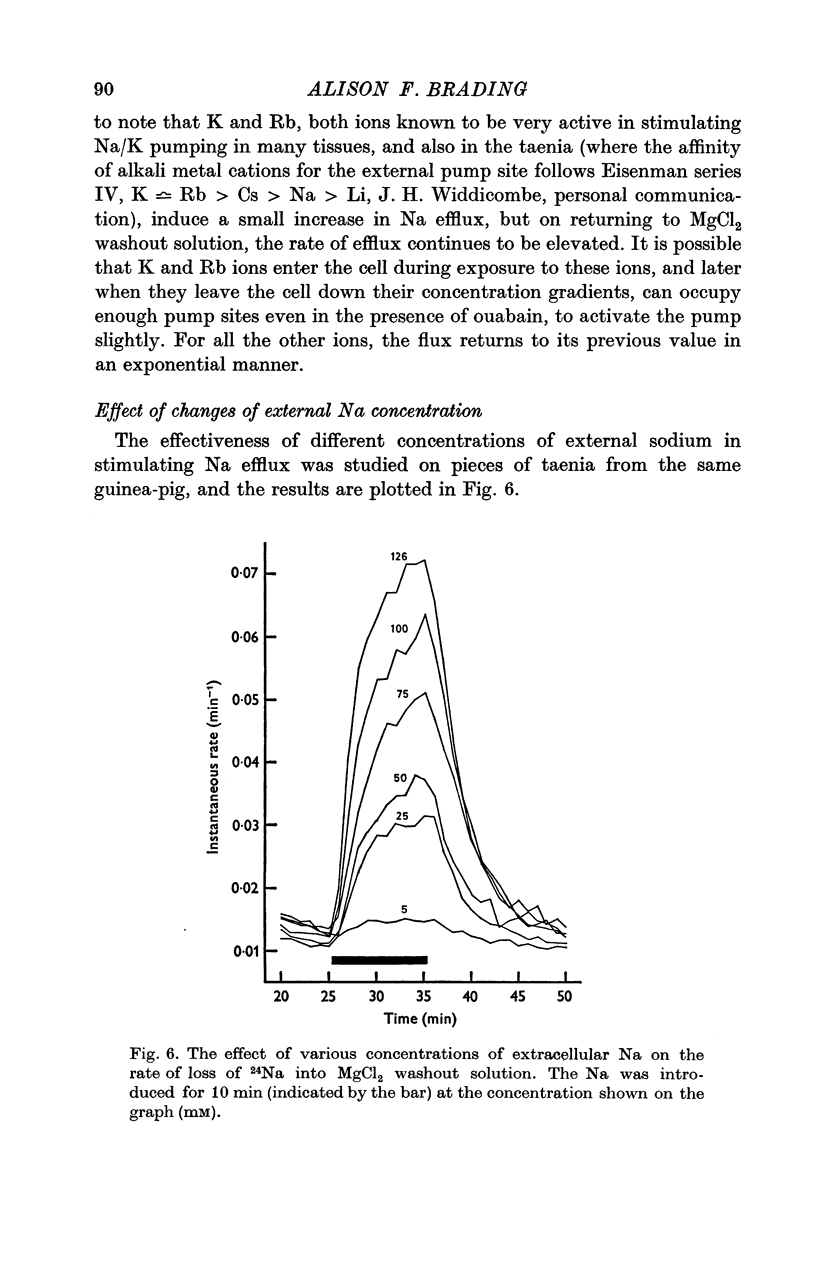
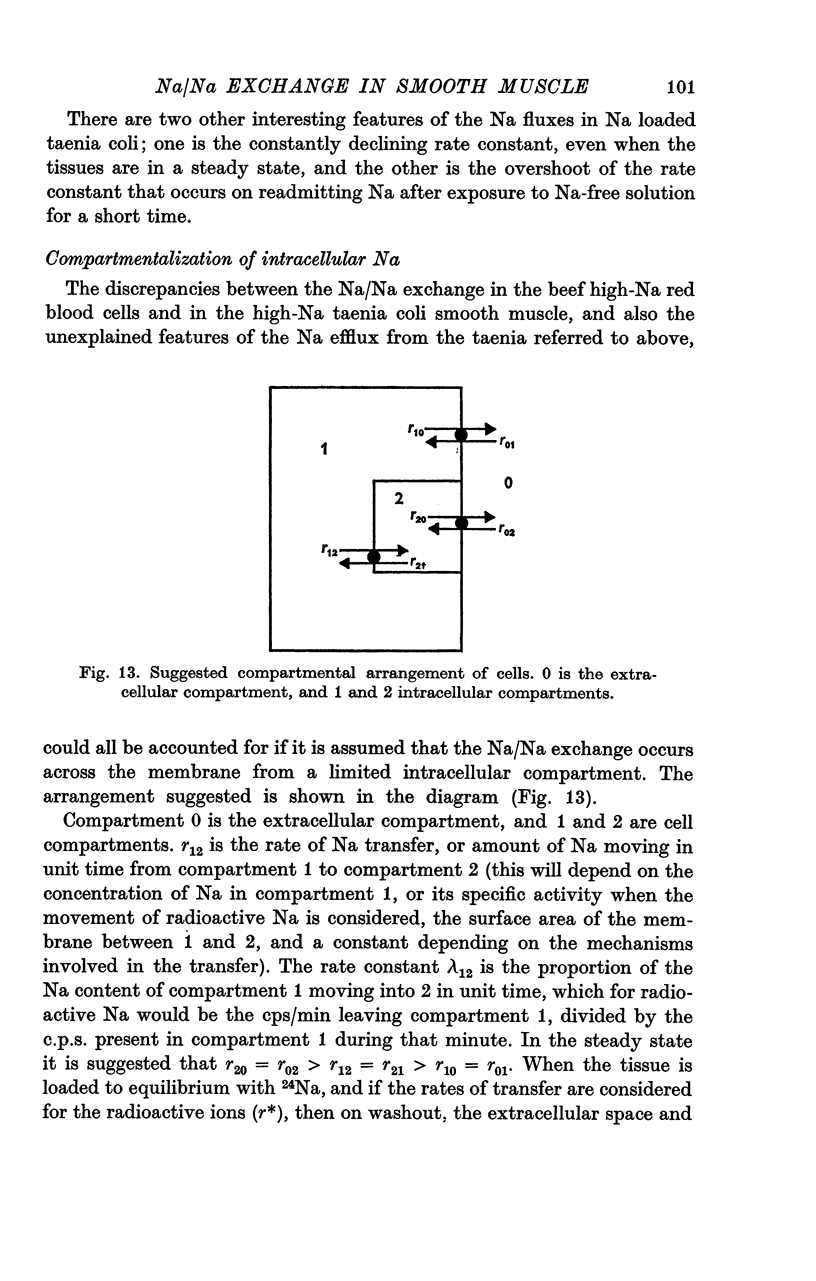
External Na has been shown to initiate a loss of 24 Na from high-Na smooth muscle of the guinea-pig taenia coli. This is an ouabain-insensitive effect, and there appears to be a 1:1 exchange of Na ions, suggesting a classical Na/Na exchange mechanism. 2. The Na/Na exchange has many properties in common with a similar exchange studied in high-Na beef erythrocytes by Motais (1973), and Motais & Sola (1973). It is very temperature-sensitive, it is partially inhibited by the sulphydryl reagents studied and it has a fairly low external affinity for Na. 3. The affinity of the external site for alkali metal cations is Na greater than Li greater than K greater than Rb greater than Cs. 4. It has proved impossible to estimate the affinity of the intracellular sites for Na. The curve relating intracellular Na content with the stimulated efflux reaches a maximum and then declines slightly. 5. Another unexpected finding was that after the rate of loss of Na has been reduced in a Na-free medium, reintroducing Na causes an overshoot in the rate, it increases to a value beyond the original one, and then slowly declines to it. 6. Unlike the classical Na/Na mechanism, the process is reduced, but not abolished by metabolic inhibition that depletes the tissue of ATP. 7. The results are interpreted to suggest that the Na/Na exchange is occurring from a cellular compartment of limited volume, which is itself exchanging with the main cell compartment. It is suggested that this small compartment is the sarcoplasmic reticulum, and the effect of metabolic inhibition is to interfere in some way with the relationship between this compartment and the cell membrane.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. C., Ramon F., Snyder A. Studies on calcium and sodium in uterine smooth muscle excitation under current-clamp and voltage-clamp conditions. J Gen Physiol. 1971 Sep;58(3):322–339. doi: 10.1085/jgp.58.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J., Holmberg B. The effects of K plus -free solution on tension development in the smooth muscle taenia coli from the guinea pig. Acta Physiol Scand. 1971 Jul;82(3):322–332. doi: 10.1111/j.1748-1716.1971.tb04973.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Analysis of the effluxes of sodium, potassium and chloride ions from smooth muscle in normal and hypertonic solutions. J Physiol. 1971 May;214(3):393–416. doi: 10.1113/jphysiol.1971.sp009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Ion distribution and the role of calcium in cellular function. Ion distribution and ion movements in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):35–46. doi: 10.1098/rstb.1973.0007. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Jones A. W. Distribution and kinetics of CoEDTA in smooth muscle, and its use as an extracellular marker. J Physiol. 1969 Feb;200(2):387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
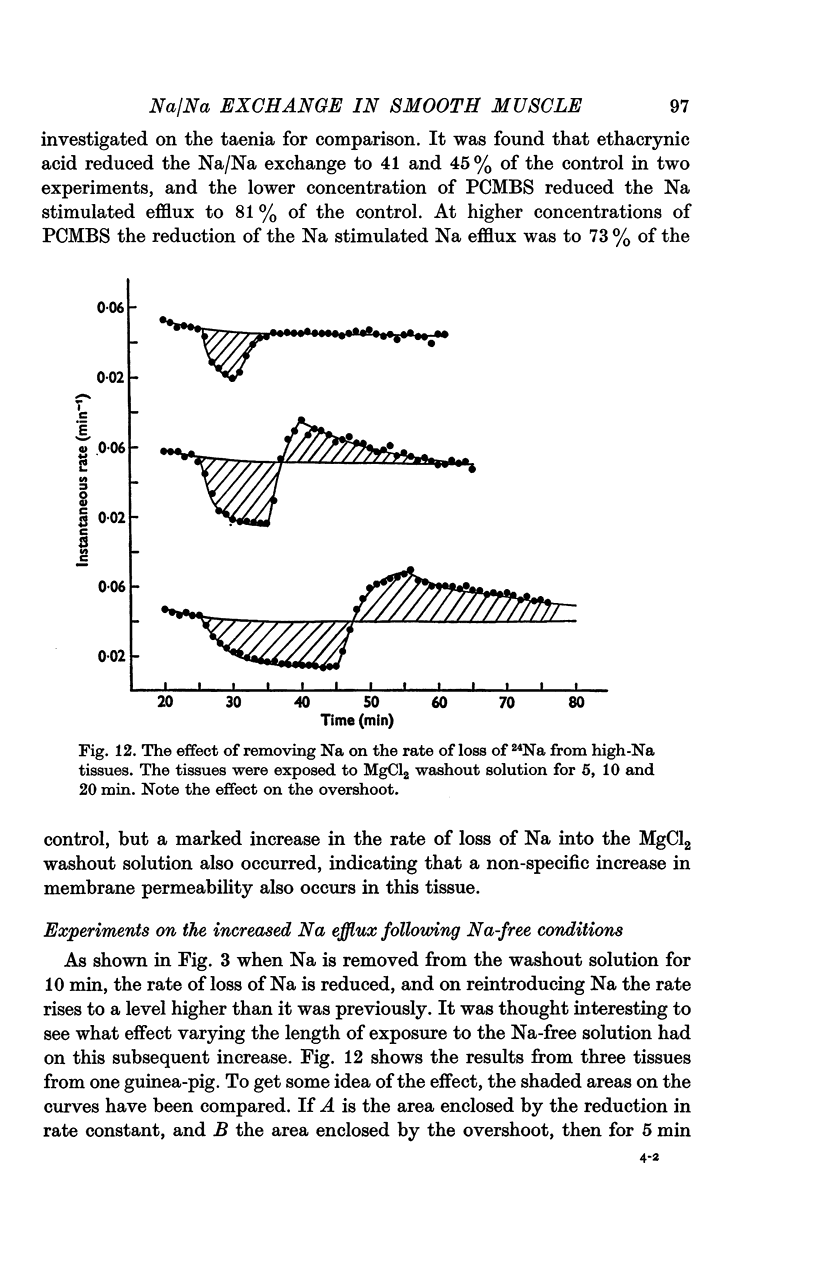
- Brading A. F. Proceedings: Na-Na exchange in guinea-pig taenia coli. J Physiol. 1974 May;239(1):35P–36P. [PubMed] [Google Scholar]
- Brading A. F., Tomita T. Ionically induced volume changes of the smooth muscle of the guinea-pig taenia coli. Experientia. 1972 May 15;28(5):521–523. doi: 10.1007/BF01931854. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Effect of sodium and sodium-substitutes on the active ion transport and on the membrane potential of smooth muscle cells. J Physiol. 1973 Feb;228(3):733–748. doi: 10.1113/jphysiol.1973.sp010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and mitochondria as cation accumulation sites in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):17–23. doi: 10.1098/rstb.1973.0005. [DOI] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Cellular structures and electrophysiological behaviour. Fine structure of smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):7–16. doi: 10.1098/rstb.1973.0004. [DOI] [PubMed] [Google Scholar]
- Gabella G. Relationship between sarcoplasmic reticulum and caveolae intracellulares in the intestinal smooth muscle. J Physiol. 1971 Jul;216(1):42P–44P. [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Steinhardt R. A. The components of the sodium efflux in frog muscle. J Physiol. 1968 Oct;198(3):581–599. doi: 10.1113/jphysiol.1968.sp008627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Kao C. Y. Electrophysiological actions of oxytocin on the rabbit myometrium. J Gen Physiol. 1969 Jun;53(6):758–780. doi: 10.1085/jgp.53.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDLEY B. D., HOSHIKO T. THE EFFECTS OF ALKALI METAL CATIONS AND COMMON ANIONS ON THE FROG SKIN POTENTIAL. J Gen Physiol. 1964 Mar;47:749–771. doi: 10.1085/jgp.47.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Armstrong W. M. State and distribution of potassium and sodium ions in frog skeletal muscle. J Membr Biol. 1974;15(4):331–362. doi: 10.1007/BF01870094. [DOI] [PubMed] [Google Scholar]
- Motais R. Sodium movements in high-sodium beef red cells: properties of a ouabain-insensitive exchange diffusion. J Physiol. 1973 Sep;233(2):395–422. doi: 10.1113/jphysiol.1973.sp010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motais R., Sola F. Characteristics of a sulphydryl group essential for sodium exchange diffusion in beef erythrocytes. J Physiol. 1973 Sep;233(2):423–438. doi: 10.1113/jphysiol.1973.sp010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Rogus E., Zierler K. L. Sodium and water contents of sarcoplasm and sarcoplasmic reticulum in rat skeletal muscle: effects of anisotonic media, ouabain and external sodium. J Physiol. 1973 Sep;233(2):227–270. doi: 10.1113/jphysiol.1973.sp010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. H. Proceedings: The effect of lanthanum on ion content and movement in the guinea-pig's taenia coli. J Physiol. 1974 Sep;241(2):106P–107P. [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


