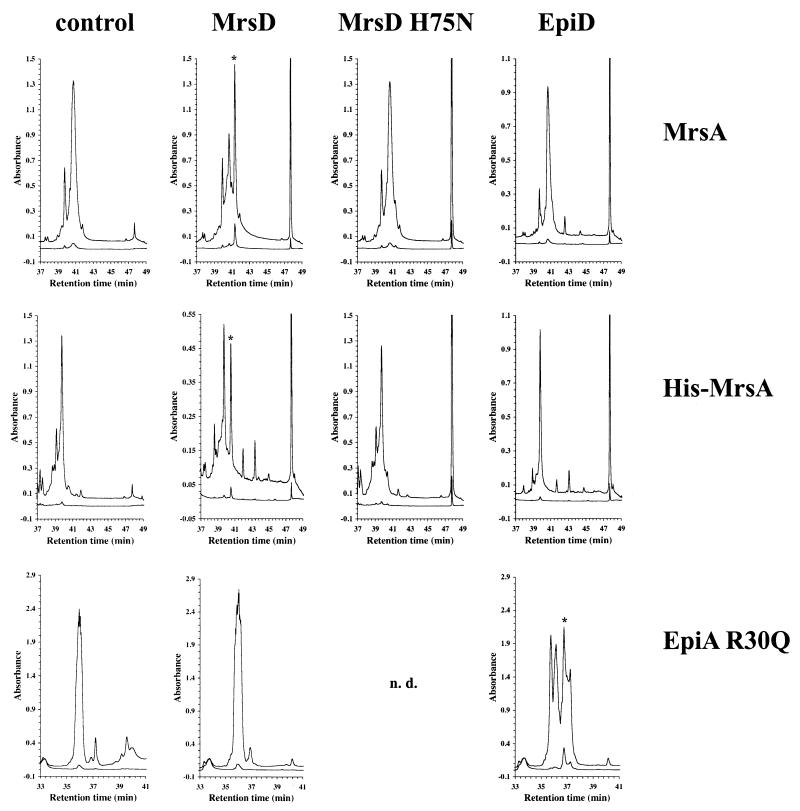
FIG. 5.
MrsD catalyzes the oxidative decarboxylation of MrsA and His-MrsA. The RPC-purified peptides MrsA (top), His-MrsA (middle), and EpiA R30Q (bottom) were incubated without any enzyme (control), with MrsD, with MrsD H75N, and with EpiD as indicated. The reaction mixtures were separated by RPC, and the elution was followed by measuring the absorbance at 214 nm (upper line), 260 nm (lower line), and 280 nm (data not shown). The LanD reaction products show an increased absorbance at 260 nm (and at 280 nm) due to the formation of the aminoenethiol chromophore and are labeled with an asterisk. The LanD reaction products are not very stable and are converted nonenzymatically to other compounds (20), as can be seen in the case of the reaction between EpiD and EpiA R30Q (positive control for the oxidative decarboxylase assay). The reaction of MrsD H75N with EpiA R30Q was not determined (n.d.) because this peptide was already not a substrate of wild-type MrsD enzyme.