Abstract
1. The rate of heat production of resting muscle is increased by hypertonic solutions.
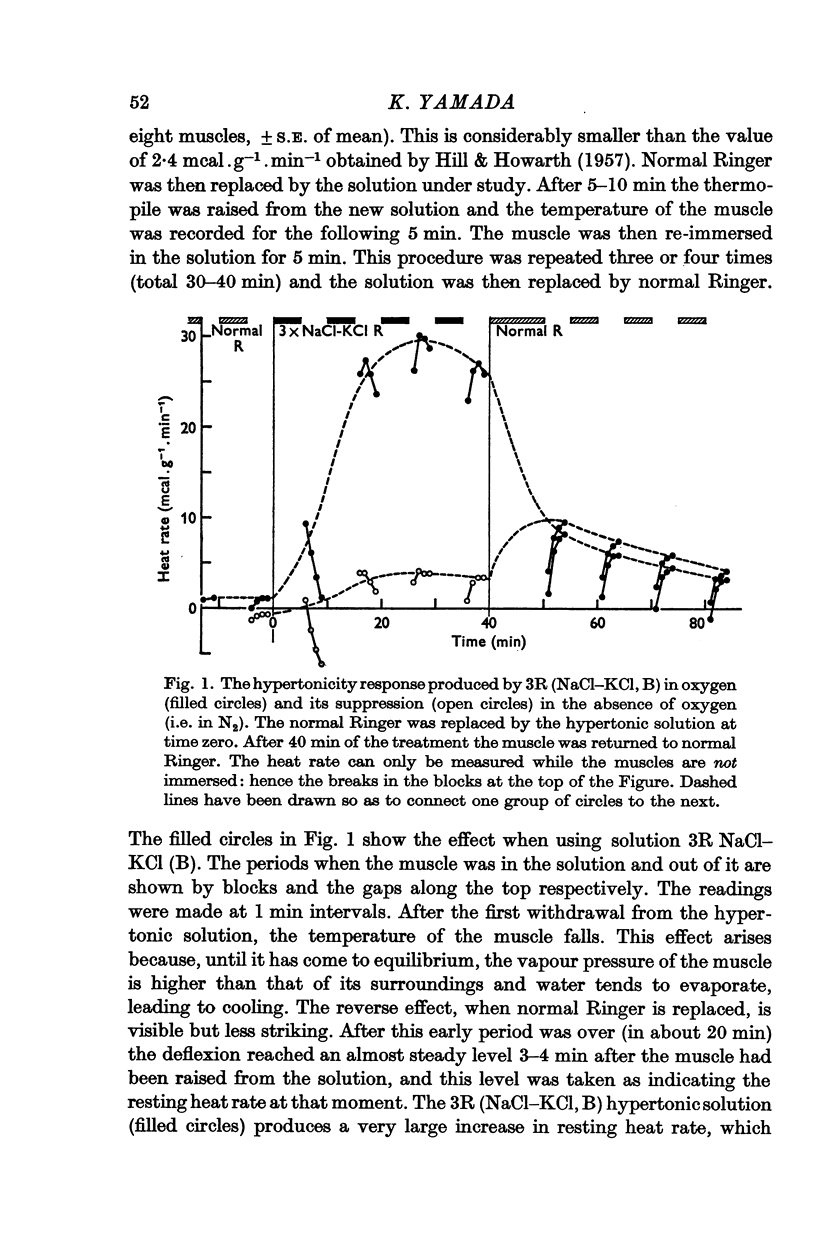
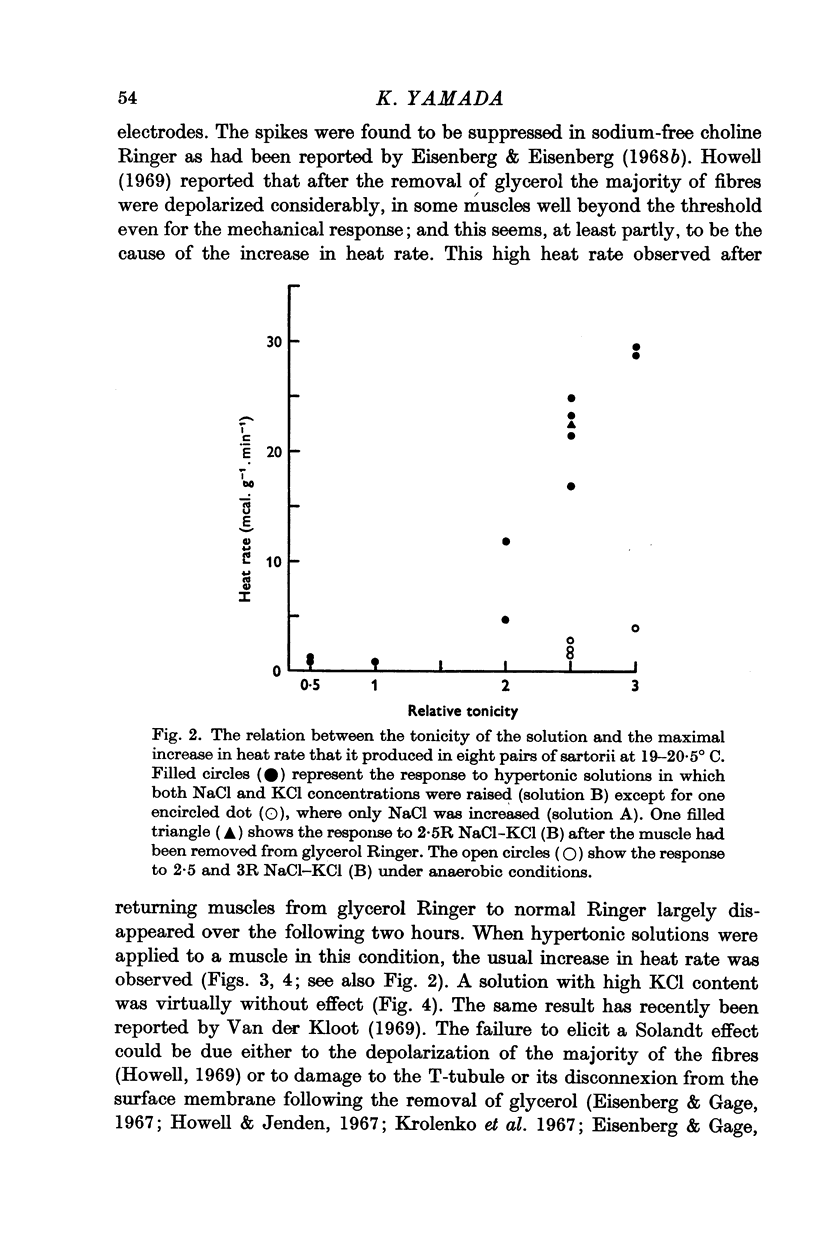
2. The threshold osmolality required to produce the increased heat rate is less than 2 times normal; at 2·5-3 times normal the heat production rises to 20-50 mcal.g-1.min-1, which is 10-20 times the basal rate.
3. In anaerobic conditions, the effect of hypertonic solutions on heat rate is only one tenth of that in aerobic conditions.
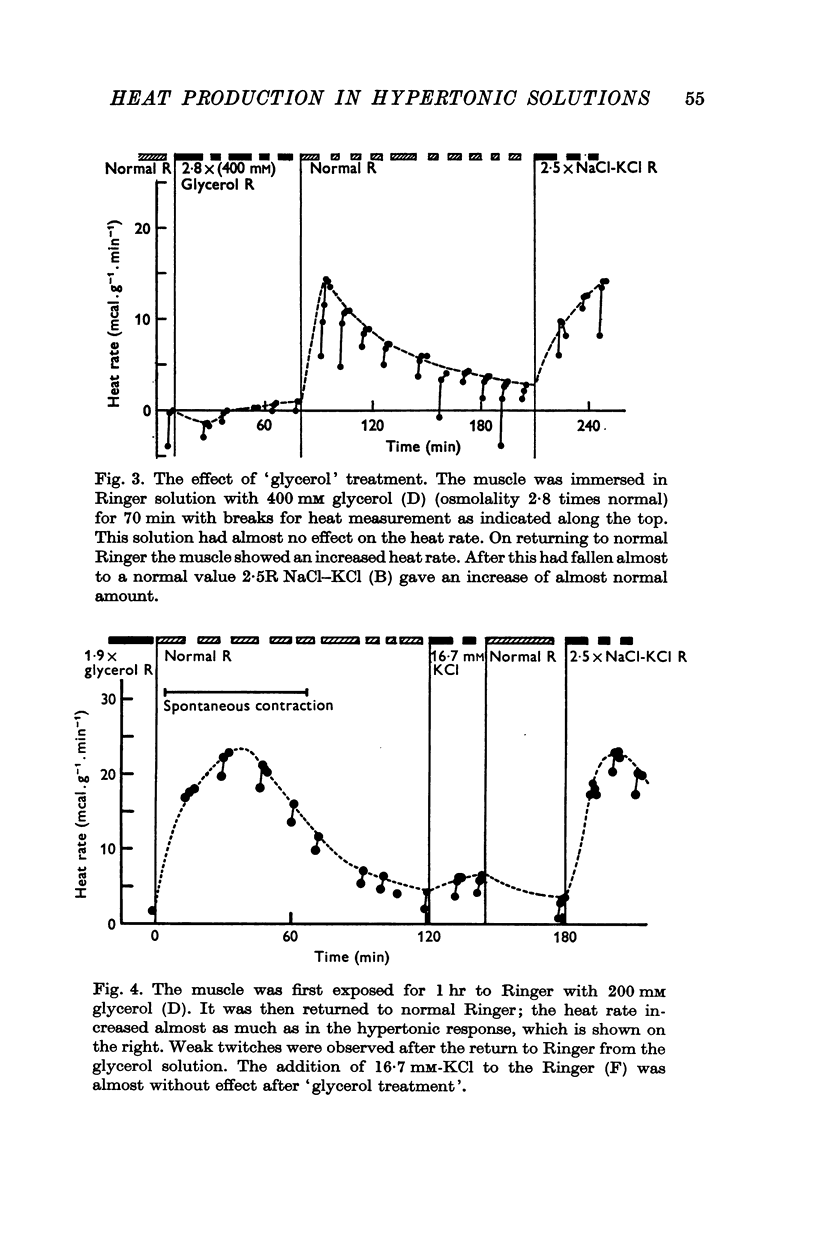
4. A glycerol-treated muscle, with damaged tubular system, still gives a normal response to hypertonic solutions, though it does not respond to raised K+ concentration.
5. The metabolic response to hypertonic solutions is considerably suppressed by procaine.
6. Ouabain, 10-5-10-4 M, has no effect.
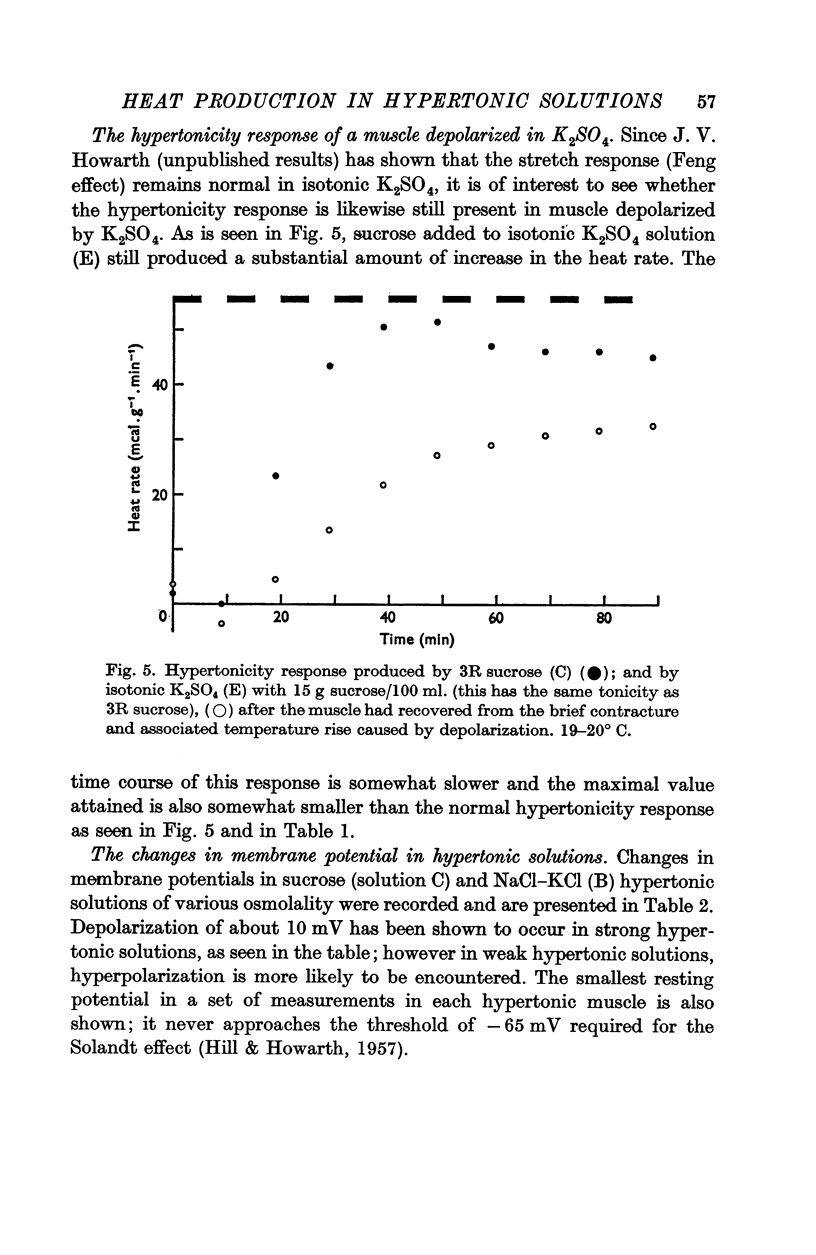
7. The response remains substantial in a muscle which has been depolarized in isotonic K2SO4.
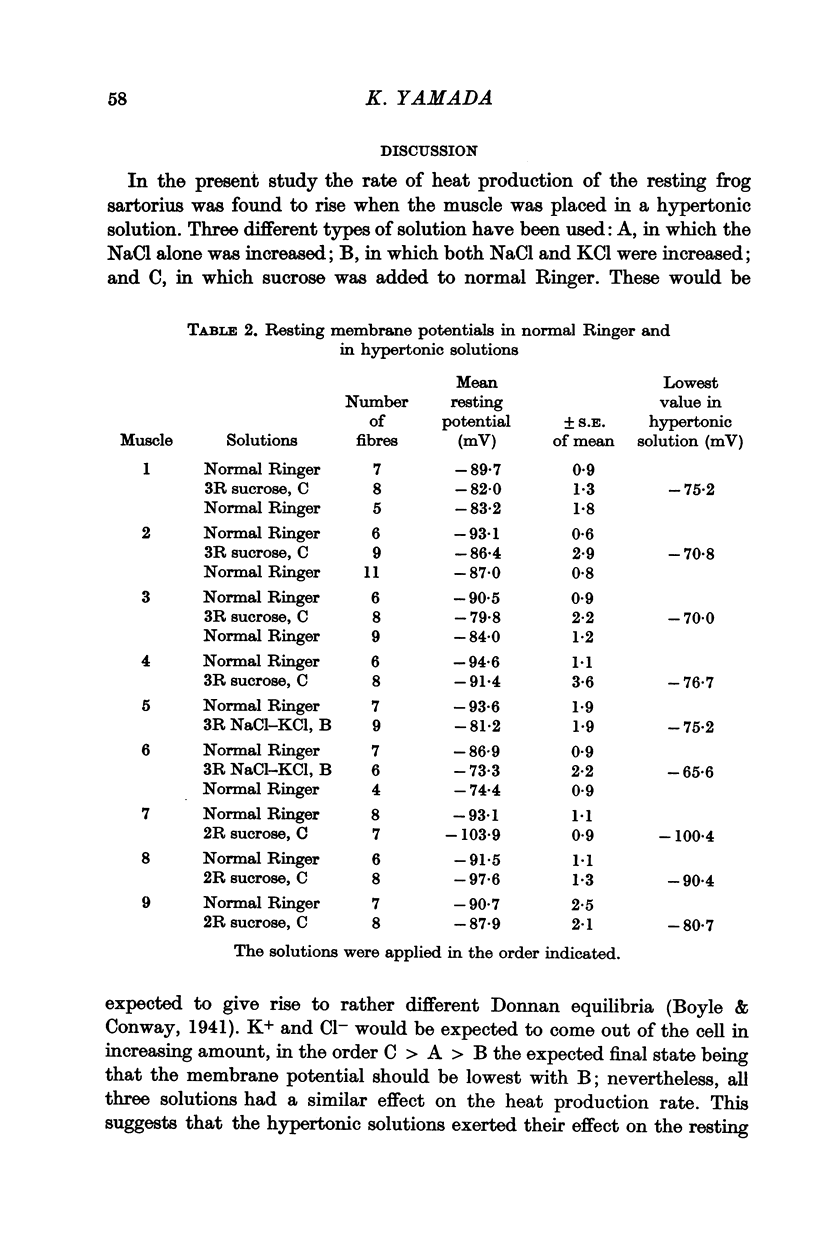
8. The membrane potential is slightly reduced by hypertonic solutions, but this cannot account for the increase of the resting metabolism.
9. It is suggested that the effect may be due to the release of calcium ions, which produce an increase in myosin ATPase activity.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birks R. I., Davey D. F. Osmotic responses demonstrating the extracellular character of the sarcoplasmic reticulum. J Physiol. 1969 May;202(1):171–188. doi: 10.1113/jphysiol.1969.sp008802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WEBER A. THE STEADY STATE OF CYTOCHROME B DURING REST AND AFTER CONTRACTION IN FROG SARTORIUS. J Physiol. 1963 Nov;169:263–277. doi: 10.1113/jphysiol.1963.sp007255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Caffeine- and potassium-induced contractures of frog striated muscle fibers in hypertonic solutions. J Gen Physiol. 1966 Sep;50(1):129–139. doi: 10.1085/jgp.50.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Volume and twitch tension changes in single muscle fibers in hypertonic solutions. J Gen Physiol. 1968 Nov;52(5):793–809. doi: 10.1085/jgp.52.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinch N. F. On the increase in rate of heat production caused by stretch in frog's skeletal muscle. J Physiol. 1968 May;196(2):397–414. doi: 10.1113/jphysiol.1968.sp008514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemers-Lambert C., Debrun F. M., Dethier G., Manil J. Métabolisme des esters phosphorés dans le sartorius de Rana temporaria traité par une solution de Ringer hypertonique. Arch Int Physiol Biochim. 1966 Jun;74(3):374–396. doi: 10.3109/13813456609059918. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., HARRIS E. J. Factors influencing the sodium movement in frog muscle with a discussion of the mechanism of sodium movement. J Physiol. 1957 Mar 11;135(3):567–580. doi: 10.1113/jphysiol.1957.sp005731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Transverse tubular system in glycerol-treated skeletal muscle. Science. 1968 Jun 14;160(3833):1243–1244. doi: 10.1126/science.160.3833.1243. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Frog skeletal muscle fibers: changes in electrical properties after disruption of transverse tubular system. Science. 1967 Dec 29;158(3809):1700–1701. doi: 10.1126/science.158.3809.1700. [DOI] [PubMed] [Google Scholar]
- FEINSTEIN M. B. INHIBITION OF CAFFEINE RIGOR AND RADIOCALCIUM MOVEMENTS BY LOCAL ANESTHETICS IN FROG SARTORIUS MUSCLE. J Gen Physiol. 1963 Sep;47:151–172. doi: 10.1085/jgp.47.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C., PEACHEY L. D. THE RELATION BETWEEN THE LATE AFTER-POTENTIAL AND THE SIZE OF THE TRANSVERSE TUBULAR SYSTEM OF FROG MUSCLE. J Gen Physiol. 1964 Nov;48:235–263. doi: 10.1085/jgp.48.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T. P. The effect of length on the resting metabolism of muscle. J Physiol. 1932 Apr 26;74(4):441–454. doi: 10.1113/jphysiol.1932.sp002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Sandow A. Caffeine-induced contracture and potentiation of contraction in normal and denervated rat muscle. Life Sci. 1965 Jun;4(11):1149–1156. doi: 10.1016/0024-3205(65)90104-9. [DOI] [PubMed] [Google Scholar]
- HARRIS E. J. An effect of stretch upon the sodium output from frog muscle. J Physiol. 1954 May 28;124(2):242–247. doi: 10.1113/jphysiol.1954.sp005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARTH J. V. The effect of hypertonic solutions on the velocity of shortening of the frog's sartorius. J Physiol. 1957 Jun 18;137(1):23–4P. [PubMed] [Google Scholar]
- Hartree W., Hill A. V. The heat production of muscles treated with caffein or subjected to prolonged discontinuous stimulation. J Physiol. 1924 May 23;58(6):441–454. doi: 10.1113/jphysiol.1924.sp002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistracher P., Hunt C. C. The effect of procaine on snake twitch muscle fibres. J Physiol. 1969 May;201(3):627–638. doi: 10.1113/jphysiol.1969.sp008776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKO N., SATO M. The effect of calcium ions on electrical properties of striated muscle fibres. Jpn J Physiol. 1957 Mar 15;7(1):51–63. doi: 10.2170/jjphysiol.7.51. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., MAISEL G. W. The energy requirement for sodium extrusion from a frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):383–392. doi: 10.1098/rspb.1954.0031. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- KREBS E. G., GRAVES D. J., FISCHER E. H. Factors affecting the activity of muscle phosphorylase b kinase. J Biol Chem. 1959 Nov;234:2867–2873. [PubMed] [Google Scholar]
- Krolenko S. A., Adamian S. Ia. Pronitsaemost' myshechnykh volokon dlia neélektrolitov. Tsitologiia. 1967 Feb;9(2):185–192. [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER M. H. Metabolic aspects of ionic shifts in toad muscle. Biochim Biophys Acta. 1962 Mar 12;57:475–494. doi: 10.1016/0006-3002(62)91156-3. [DOI] [PubMed] [Google Scholar]
- MULLER M., SIMON S. E. A comparison of ion shifts with respiration and glycolysis in muscle. Biochim Biophys Acta. 1960 Jan 1;37:107–119. doi: 10.1016/0006-3002(60)90084-6. [DOI] [PubMed] [Google Scholar]
- NOVOTNY I., VYSKOCIL F., VYKLICKY L., BERANEK R. Potassium and caffeine induced increase of oxygen consumption in frog muscle and its inhibition by drugs. Physiol Bohemoslov. 1962;11:277–284. [PubMed] [Google Scholar]
- Nakajima S., Nakajima Y., Peachey L. D. Speed of repolarization and morphology of glycerol-treated muscle fibres. J Physiol. 1969 Feb;200(2):115P–116P. [PubMed] [Google Scholar]
- Ozawa E., Ebashi S. Requirement of Ca ion for the stimulating effect of cyclic 3',5'-AMP on muscle phosphorylase b kinase. J Biochem. 1967 Aug;62(2):285–286. doi: 10.1093/oxfordjournals.jbchem.a128663. [DOI] [PubMed] [Google Scholar]
- PAGE E., GOERKE R. J., STORM S. R. CAT HEART MUSCLE IN VITRO. IV. INHIBITION OF TRANSPORT IN QUIESCENT MUSCLES. J Gen Physiol. 1964 Jan;47:531–543. doi: 10.1085/jgp.47.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solandt D. Y. The effect of potassium on the excitability and resting metabolism of frog's muscle. J Physiol. 1936 Feb 8;86(2):162–170. doi: 10.1113/jphysiol.1936.sp003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., WINICUR S. The role of calcium in the superprecipitation of actomyosin. J Biol Chem. 1961 Dec;236:3198–3202. [PubMed] [Google Scholar]
- van der Kloot W. The steps between depolarization and the increase in the respiration of frog skeletal muscle. J Physiol. 1969 Oct;204(3):551–569. doi: 10.1113/jphysiol.1969.sp008931. [DOI] [PMC free article] [PubMed] [Google Scholar]


