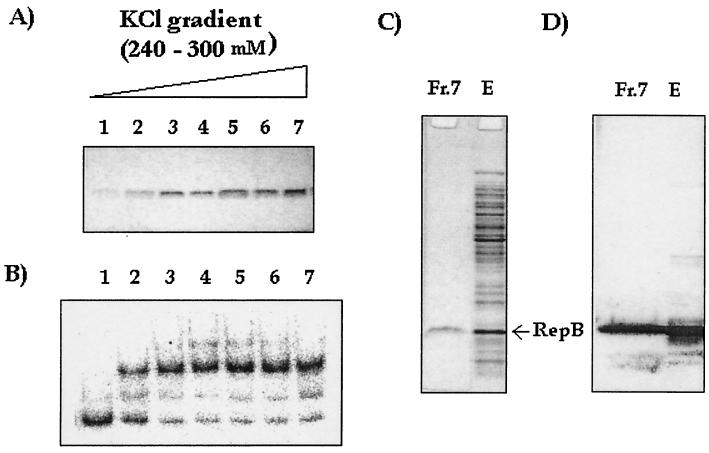
FIG. 4.
Purification of RepB expressed from pTAB2 with a Mono S column. The active fractions were eluted by using a 240 to 300 mM KCl gradient in buffer A. (A) SDS-PAGE analysis of eluted fractions. (B) Binding activity after dialysis against low-salt buffer (50 mM KCl in buffer A). (C) SDS-PAGE analysis of fraction 7 (lane Fr.7) and the unfractionated extract (lane E). Lane Fr.7 contained 1 μg of fraction 7, and lane E contained 10 μg of the extract. (D) Corresponding Western blot with anti-RepB antiserum.