Abstract
The T–786C promoter and 27-bp repeat intron 4 polymorphisms in the endothelial NO synthase (eNOS) gene have been inconsistently associated with various eNOS-related phenotypic changes. We explored molecular mechanisms underlying the inconsistency. We constructed pGL3 luciferase reporter vectors by inserting an eNOS promoter fragment containing either T or C nucleotide at −786 bp at the 5′ end of the luciferase coding region and eNOS intron 4 containing either 5× or 4×27-bp repeats at the 3′ end of the luciferase gene. The transcription efficiency in the T promoter was lower than in the C promoter (15.7±1.0% vs 83.3±5.8%, P<0.01 when 5×27-bp was an enhancer and 37.6±4.7% vs 58.9±7.5%, P<0.01 when 4×27bp was an enhancer). Although cigarette smoking extracts treatment increased the transcription efficiency significantly in the T promoter (1.7-fold, P<0.01), it reduced the C promoter efficiency (by 10% to 15%). A mobility shift assay revealed positive binding of the 27-bp repeat fragment with endothelial cell nuclear protein extracts. Our study demonstrates a cis-acting role of the 27-bp repeats in eNOS promoter function and a haplotype-specific expression pattern determined by DNA variants at −786 bp and intron 4 of the eNOS gene that is also modifiable by cigarette smoking.
Keywords: gene, gene regulation, smoking, haplotypes, endothelial NO synthase
Endothelial dysfunction is a key step in both initiation and progression of atherogenesis.1 Many factors, such as cigarette smoking, can cause endothelial damage severe enough to be followed by atherosclerotic or thrombotic changes. Among many endogenous protectors, endothelial NO synthase (eNOS) has been recognized as most important. Not only are dysfunctional endothelial cells often accompanied by depressed eNOS activity,2 animals with eNOS deficiency induced by eNOS gene knockout techniques are also more susceptible to atherogenic challenges.3–5
Although we have not yet detected any human subject completely lacking eNOS expression or activity, we can logically predict that humans with defective eNOS gene expression would also be prone to atherogenesis. One of the strategies to demonstrate such relationship is to explore relationships between the DNA variants in the eNOS gene and vascular diseases. However, results from a large number of population studies conducted so far have been inconsistent and often contradictory.6 – 8 Among studied polymorphisms, the T–786C, 27-bp repeats at intron 4 and the G894T variants have received the most attention.6 The G894T at exon 7 results in a Glu to Asp change and is suggested to increase the susceptibility to cleavage,9 although others contend that this is as an in vitro acidic hydrolysis.10 However, many epidemiological studies at the population and tissue levels point to the possibility that the T–786C and the 27-bp repeat in intron 4 (either 4× or 5× repeats in whites), which are in close linkage disequilibrium, may be functional in quantitative eNOS regulation.6 – 8,11,12 Indeed, the T–786C substitution was shown to affect promoter efficiency.13
We hypothesize that in conjunction with the eNOS promoter, the intron 4 may have a cis-acting effect on eNOS transcription. The 27-bp repeat could bind with nuclear proteins as an enhancer/repressor to promote/ suppress the transcription efficiency. The functional significance of intron 4 could be conditional on the presence of the eNOS promoter. The 27-bp in intron 4 of the eNOS gene fits the characteristic pattern of an enhancer, which is typically a repeat sequence fragment.14
In the current study, we explored the independent and conjoint effects of the 27-bp repeat with the eNOS promoter T–786C variant on transcription efficiency in a pGL3 reporter system. We evaluated direct effect of cigarette smoking on eNOS promoter and intron 4 in relation to transcription efficiency. We also examined the possible transcription factors which may bind to the 27-bp fragment.
Methods
Polymerase Chain Reaction (PCR) Cloning of the eNOS Promoter and Intron 4 Into the pGL3 Luciferase Reporter Vectors
To explore the effects of the 27-bp repeats in eNOS intron 4 on transcription efficiency, we generated 10 constructs. Both the promoter region (from −1302 bp to +163 bp) and the 27-bp repeats in intron 4 (+5111 bp to +5483 bp) of the eNOS gene were amplified by PCR and purified with a QIAGEN column purification method (Qiagen, Pty Ltd). The genomic DNA from individuals with different eNOS genotypes obtained in our previous studies were used as DNA templates for cloning.15,16 The promoter fragment of either T or C at the −786-bp position (named T or C promoter) was inserted into a promoterless pGL3-luciferase reporter gene basic vector. The sites of insertion at +43 (XhoI site) and +53 (HindIII site) were selected for directional cloning. To assess the cis-acting effect of the 27-bp repeat, we inserted the T or C promoter at the 5′ end of the luciferase coding sequence in the basic pGL3 vector (promoterless and enhancerless). The 27-bp fragments were then inserted at the 3′ region of the luciferase coding sequence. The sites of insertion according to the sequence of pGL3-basic vector were at +2004 (BamHI site) and +2010 (SalI site). Vectors containing eNOS promoter and/or 27-bp repeat inserts were subject to direct PCR sequencing for confirmation of both orientation and corrected DNA inserts by using an ABI377 auto sequencer (Perkin-Elmer).
Human Endothelial Cell Culture, Transfection, and Expression
Human umbilical venous endothelial cells were harvested from the umbilical vein by using collagenase digestion. Cells were cultured up to 4 to 6 passages in Ham’s F12K medium (American Type Culture Collection) supplemented with 20% FCS, 100 μg/mL heparin, and 45 μg/mL endothelial cell growth factor in gelatin-coated culture flask, at 5% CO2, 37°C. FuGENE 6 Transfection Reagent (Roche Molecular Biochemicals) was used to transfer the plasmid into endothelial cells. The transfection efficiency with the FuGENE 6 reagent was between 10% and 15% in our laboratory. Each assay was conducted in triplicates and repeated 3 times. The mean±SEM percentage of luciferase activity compared with positive control vector (SV40 promoter and enhancer) was presented for each eNOS containing vector.
Electrophoresis Mobility Shift Assay
We used a nonradioisotope method with digoxigenin (DIG)-labeled oligonucleotide in the binding assay. A double-strand DNA fragment containing the 27-bp repeat (5′-GAA GTC TAG ACC TGC TGC AGG GGT GAG-3′) was oligosynthesized and labeled with DIG by using a 3′ end-labeling method (Roche). The endothelial nuclear proteins were extracted by using a kit method (GENEKA Biotechnology Inc). The binding reaction between the nuclear extracts and DIG-labeled oligonucleotide were conducted at room temperature for 20 minutes in the presence of poly(dI-dC). The reaction solution was electrophoresed in 6% native polyacrylamide gel electrophoresis for 90 minutes at 64 V (8 V/cm gel) at 4°C. The gel was then transblotted onto positively charged nylon membrane (Roche), which reacted with anti-DIG-alkaline phosphatase conjugate before the chemiluminescent detection by using disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5′-chloro) tricyclo [3.3.1.13,7] decan}-4-yl)phenyl phosphate as substrate.
Preparation of Cigarette Smoking Extracts (CSE)
The CSE was prepared by using a suction syringe in which one cigarette was puffed directly into 20 mL of culture media. The standard research cigarette (1R3F, University of Kentucky) was inserted into a 3-cm length of plastic tubing that was attached to a Pasteur pipette submerged in a filtering flask containing 20 mL of Ham’s F12K medium. A fixed negative pressure generated by the syringe was applied to the flask through a separate tube so that the ignited cigarette was completely consumed in 5 minutes. The concentration of CSE was calculated in arbitrary units as cigarette equivalents/mL medium, eg, 1 cigarette bubbled through 20 mL of medium yields 0.01 cigarette equivalent/mL medium. The CSEs were used for treatment within 15 minutes of extraction. The direct effect of CSEs on transcription efficiency was assessed by treating transfected endothelial cells with a dose of CSE for a period up to 24 hours. We selected a dose of 0.01 cigarette equivalents/mL in culture medium, which was shown not to cause macroscopic endothelial cell death within 24 hours.
Results
The Effect of T→C Transition at −786 bp of the eNOS Gene on Transcription
The luciferase enzyme activity produced by each construct was expressed as the percentage of luciferase activity of the positive control pGL3 construct (containing SV40 promoter and enhancer). As shown in Figure 1a, the luciferase activity was significantly higher in endothelial cells transfected with the T promoter (45.2±2.2%) than that in the C promoter (28.9±2.9%, P<0.001). When the transfected endothelial cells were exposed to CSE, we observed a significantly (P<0.01) increased transcription efficiency in both promoters (T promoter, 78.5±3.8%; and the C promoter, 45.4±3.2%). The increase in transcription efficiency was slightly higher in the T promoter (1.74-fold) than that in the C promoter (1.57-fold).
Figure 1.
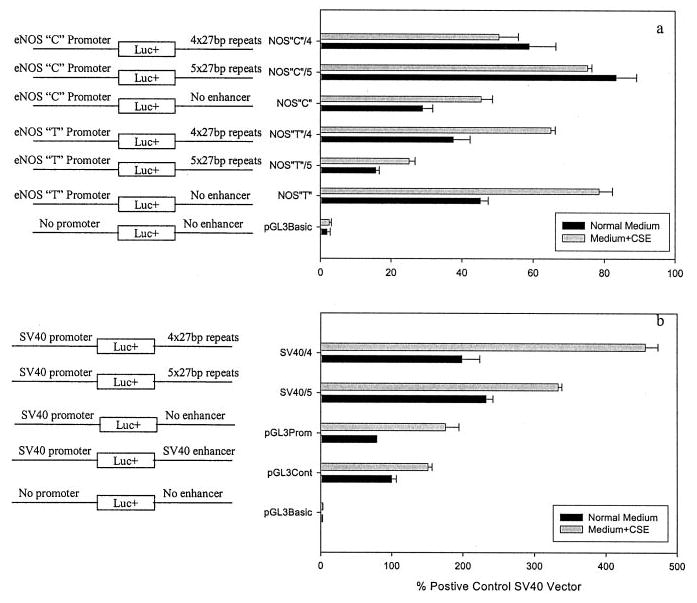
Effects of the 27-bp repeats of eNOS intron 4 on transcription efficiencies driven by eNOS promoter (a) or heterologous SV40 promoter (b). The pGL3 vectors were constructed as outlined on the left side of the figure. The 4× or 5×27-bp repeats were inserted at the place of SV40 enhancer. The constructed pGL3 vectors were transfected into human umbilical venous endothelial cells, which were cultured with or without CSE supplement for 24 hours before luciferase activity was analyzed. The transcription efficiencies were expressed as the mean±SEM (n=9) percentage of luciferase activity produced by positive control vector, ie, pGL3 vector with SV40 promoter and enhancer.
The 27-bp–Repeats in eNOS Intron 4 Acts as a cis-Acting Element in eNOS Regulation
To assess whether the 27-bp repeat in intron 4 of the eNOS gene contributes to transcription efficiency of the eNOS promoter, we inserted the 27-bp fragments containing either 4× or 5× 27-bp repeats at the 3′ end of the luciferase-coding region (Figure 1a). At the 5′ end of the luciferase coding sequence, we inserted either the eNOS T or C promoter segment. For the common T promoter, the addition of 5×27-bp repeats at the 3′ end repressed the transcription efficiency by more than 65% compared with the vector without the 27-bp repeat fragment (P<0.001, Figure 1a). Insertion of the 4×27-bp resulted in a modest reduction in the transcription efficiency (17%, P= not significant). The T promoter vectors containing either 4× or 5×27-bp inserts responded to CSE by increased transcription efficiency (1.7-fold for 4×27-bp and 1.6-fold for 5×27-bp repeats).
In contrast to the T promoter, insertion of the 5×27-bp in eNOS C promoter vectors increased rather than decreased the transcription efficiency by 2.9-fold (P<0.01, Figure 1a). The increase by the 4×27-bp insertion was ≈2-fold (P<0.01, Figure 1a). Compared with the T promoter with the 27-bp insertion, the C promoter vectors with either 4× or 5×27-bp repeat at the 3′ end region had a reduced transcription efficiency when exposed to CSE (15.4% reduction for 4×27-bp and 10.2% reduction for 5×27-bp repeat).
Furthermore, replacement of the SV40 enhancer by either 4× or 5× 27-bp repeats in vectors containing heterologous SV40 promoter at the 5′ end of the luciferase coding region also resulted in an increased transcription efficiency as we observed for the eNOS C promoter (P<0.01, Figure 1b). The increase tended to be higher for the 5× repeats than the 4× repeats. However, contrary to the eNOS C promoter in which CSE seemed to have no effect on transcription efficiency (Figure 1a), CSE treatment led to a significant increase in transcription efficiency (P<0.01, Figure 1b) in SV40 promoter vectors as we observed for the T promoter insert (Figure 1a).
The 27-bp Fragment Binds Endothelial Nuclear Proteins
Using electrophoresis mobility shift assay, we demonstrated that there was binding between endothelial nuclear proteins and the 27-bp repeat fragment of the eNOS intron 4 (Figure 2). This binding was specifically inhibited by unlabeled double-strand 27-bp repeat oligonucleotide. A database search revealed only two potential consensus transcription factor alkaline phosphatase-4 (-CCAGCTGCGG-) and TGT3 (-GACCTG-) binding sequences within the 27-bp fragment. However, individual base or group mutagenesis at both sites did not alter the binding pattern between the 27-bp fragment and the nuclear proteins.
Figure 2.
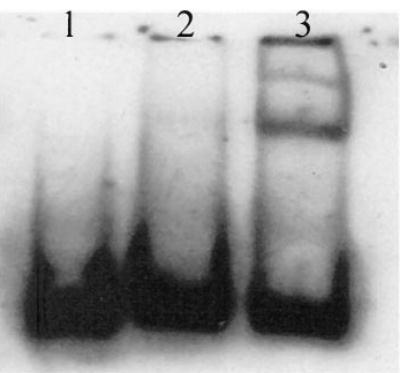
Gel electrophoresis (6% native polyacrylamide gel electrophoresis) for mobility shift assay. Lane 1: no crude nuclear extract in the binding assay as a control; Lane 2: DIG-labeled 27-bp oligonucleotide (≈3 pmol) reacted with endothelial nuclear extracts (2 μg) in the presence of 100-fold more unlabeled 27-bp oligonucleotide; Lane 3: DIG-labeled oligonucleotide (≈3 pmol) reacted with endothelial cell nuclear extracts (2 μg) in the absence of an unlabeled competitor.
Discussion
The most important findings in the current study are that not only the 27-bp repeat from the eNOS intron 4 has a cis-regulating effect on the eNOS promoter, but that the regulation also appears to be haplotype-dependent on both the eNOS promoter and intron 4 DNA sequence variants. The wild-type T promoter had 1.6-fold higher transcription efficiency than the rare C promoter (P<0.001) when the 27-bp repeat was not present. This is consistent with the findings by Nakayama et al.13 However, the presence of the 5×27-bp repressed the transcription efficiency of the T promoter to ≈35% of the original level. In contrast, the 5×27-bp increased the transcription efficiency of the C promoter to 288% (or 2.88-fold) of the original level. The rare 4×27-bp repeats had similar actions on both the T and C promoters, but to a lesser extent.
If we extrapolate this in vitro finding into the in vivo human phenotypic expression, ie, eNOS levels, it would seem that homozygotes with the T/T at −786 bp and the 5/5 repeats at intron 4 would have nearly 4-fold lower eNOS levels than those in the C/C and 4/4 homozygotes. However, end results for heterozygotes become much more complicated and would depend on haplotypes of these two sites. Based on all possible haplotypes with each given genotype at either polymorphic site, we can hypothetically predict the arbitrary levels of eNOS by taking the summation of each haplotype, assuming that the contribution of each allele to the final eNOS production is equal. For example, T/C homozygote at −786 bp could have either 75.1±6.6% or 121.0±11.0% eNOS levels when the genotype at intron 4 was 5/4 repeat heterozygote, or 99.0±7.6% (P<0.05) when the intron 4 genotype was 5× repeat homozygote. The same situation would also be true for the intron 4 polymorphism. A common 5× repeat homozygote could be associated with 31.4±3% eNOS levels when the promoter genotype was T/T, 166±9% when the promoter genotype was C/C, or 97.1±11.4% (P<0.01) for T/C heterozygote. Cigarette smoking has further complicated the genotype-phenotype relationships because cigarettes tend to regulate the expression efficiency in a haplotype-dependent fashion (Figure 1a).
In conclusion, we have demonstrated a functional 27-bp repeat at intron 4 of the eNOS gene which coordinates with the T–786C variant at the promoter region and regulates transcription efficiency in a haplotype-specific fashion. The effect of cigarette smoking on eNOS expression is further modified by the haplotypic patterns of the eNOS promoter and intron 4. Our study provides explanations at the molecular level for inconsistent findings of associations between various eNOS polymorphic markers and vascular diseases. We suggest that haplotype-based association studies may be more appropriate than single polymorphic genotype–based studies, which are often misleading. Recent technical development in experimentally measuring haplotypes,17–19 or computational estimation by the maximum likelihood method,20 would facilitate the haplotype determination in association studies of unrelated individuals. Alternatively, we could measure all DNA variants at and/or near the target candidate gene to cover possible inter-locus interactions. The advantage of this multi-locus approach is not only the wide availability of high throughput single nucleotide polymorphisms (SNPs)-typing techniques, but also it avoids the presumption for the relationships among polymorphic loci that may not be in cis-regulatory interactions.
Acknowledgments
This study was supported by National Institutes of Health grant R01 HL66053-01A1.
References
- 1.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 2.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- 3.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 4.Huang PL. Mouse models of nitric oxide synthase deficiency. J Am Soc Nephrol. 2000;11(suppl 16):S120–S123. [PubMed] [Google Scholar]
- 5.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 6.Wang XL, Wang J. Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol Genet Metab. 2000;70:241–251. doi: 10.1006/mgme.2000.3033. [DOI] [PubMed] [Google Scholar]
- 7.Kinlay S, Libby P, Ganz P. Endothelial function and coronary artery disease. Curr Opin Lipidol. 2001;12:383–389. doi: 10.1097/00041433-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wattanapitayakul SK, Mihm MJ, Young AP, Bauer JA. Therapeutic implications of human endothelial nitric oxide synthase gene polymorphism. Trends Pharmacol Sci. 2001;22:361–368. doi: 10.1016/s0165-6147(00)01692-8. [DOI] [PubMed] [Google Scholar]
- 9.Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc Natl Acad Sci U S A. 2000;97:2832–2835. doi: 10.1073/pnas.97.6.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairchild TA, Fulton D, Fontana JT, Gratton JP, McCabe TJ, Sessa WC. Acidic hydrolysis as a mechanism for the cleavage of the glu298right-arrow asp variant of human endothelial nitric-oxide synthase. J Biol Chem. 2001;276:26674–26679. doi: 10.1074/jbc.M103647200. [DOI] [PubMed] [Google Scholar]
- 11.Tanus-Santos JE, Desai M, Flockhart DA. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics. 2001;11:719–725. doi: 10.1097/00008571-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hingorani AD. Polymorphisms in endothelial nitric oxide synthase and atherogenesis: John French Lecture 2000. Atherosclerosis. 2001;154:521–527. doi: 10.1016/s0021-9150(00)00699-7. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Kugiyama K, Ogawa H, Motoyama T, Saito Y, Ogawa Y, Miyamoto Y, Nakao K. T-786–>C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999;99:2864–2870. doi: 10.1161/01.cir.99.22.2864. [DOI] [PubMed] [Google Scholar]
- 14.Lewin B. Enhancers contain bidirectional elements that assist initiation. In: Lewin B, ed. Genes VII Oxford, England: Oxford University Press; 2000:637–640.
- 15.Sim AS, Wang J, Wilcken D, Wang XL. MspI polymorphism in the promoter of the human endothelial constitutive NO synthase gene in Australian Caucasian population. Mol Genet Metab. 1998;65:62. doi: 10.1006/mgme.1998.2741. [DOI] [PubMed] [Google Scholar]
- 16.Wang XL, Sim AS, Badenhop RF, McCredie RM, Wilcken DE. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat Med. 1996;2:41–45. doi: 10.1038/nm0196-41. [DOI] [PubMed] [Google Scholar]
- 17.Yan H, Papadopoulos N, Marra G, Perrera C, Jiricny J, Boland CR, Lynch HT, Chadwick RB, de la Chapelle A, Berg K, Eshleman JR, Yuan W, Markowitz S, Laken SJ, Lengauer C, Kinzler KW, Vogelstein B. Conversion of diploidy to haploidy. Nature. 2000;403:723–724. doi: 10.1038/35001659. [DOI] [PubMed] [Google Scholar]
- 18.Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, Arnold K, Ruano G, Liggett SB. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens JC, Schneider JA, Tanguay DA, Choi J, Acharya T, Stanley SE, Jiang R, Messer CJ, Chew A, Han JH, Duan J, Carr JL, Lee MS, Koshy B, Kumar AM, Zhang G, Newell WR, Windemuth A, Xu C, Kalbfleisch TS, Shaner SL, Arnold K, Schulz V, Drysdale CM, Nandabalan K, Judson RS, Ruano G, Vovis GF. Haplotype variation and linkage disequilibrium in 313 human genes. Science. 2001;293:489–493. doi: 10.1126/science.1059431. [DOI] [PubMed] [Google Scholar]
- 20.Excoffier L, Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol. 1995;12:921–927. doi: 10.1093/oxfordjournals.molbev.a040269. [DOI] [PubMed] [Google Scholar]


