Abstract
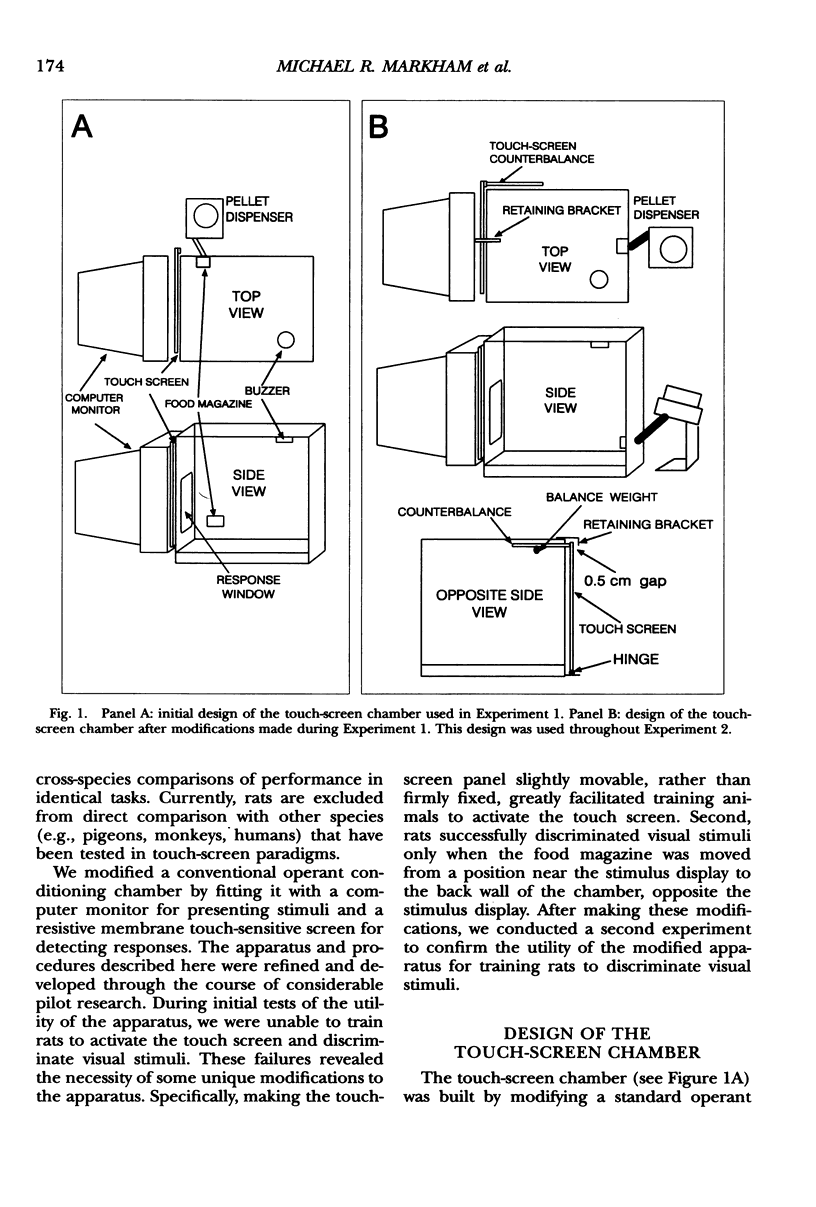
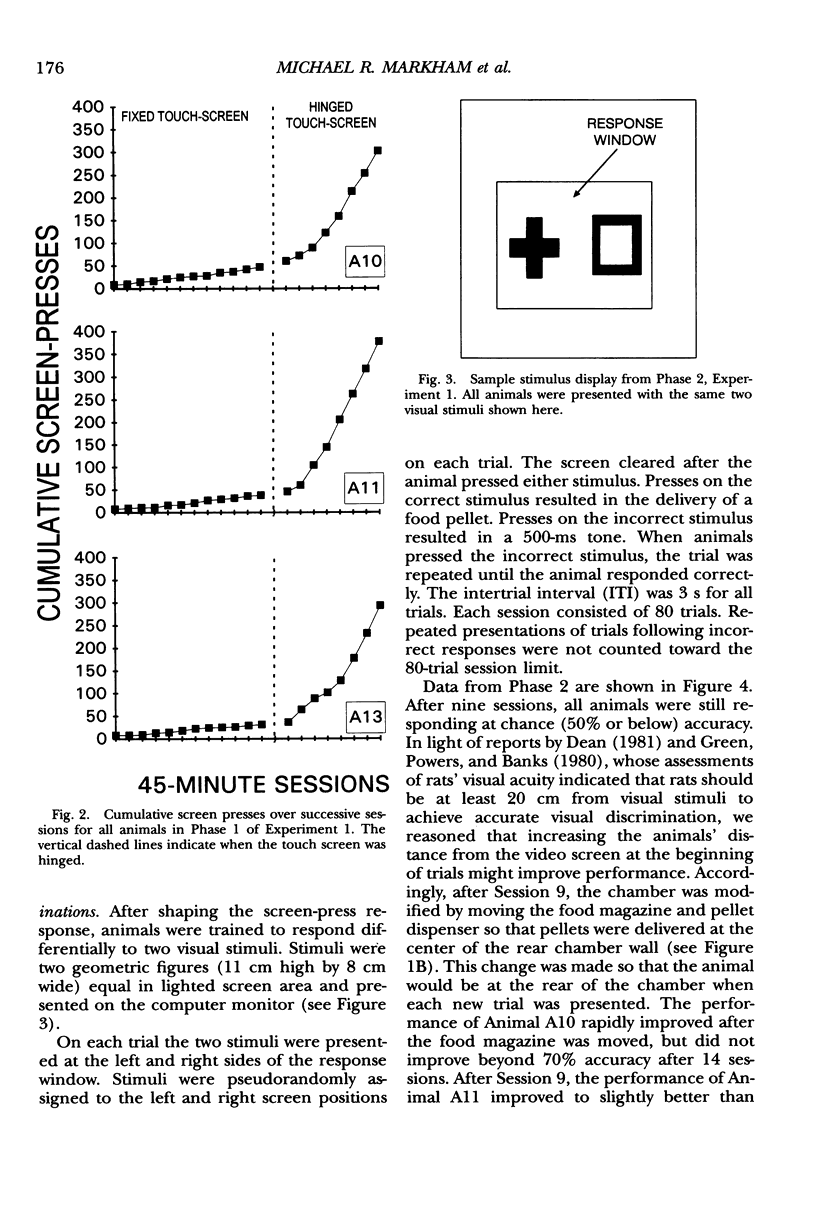
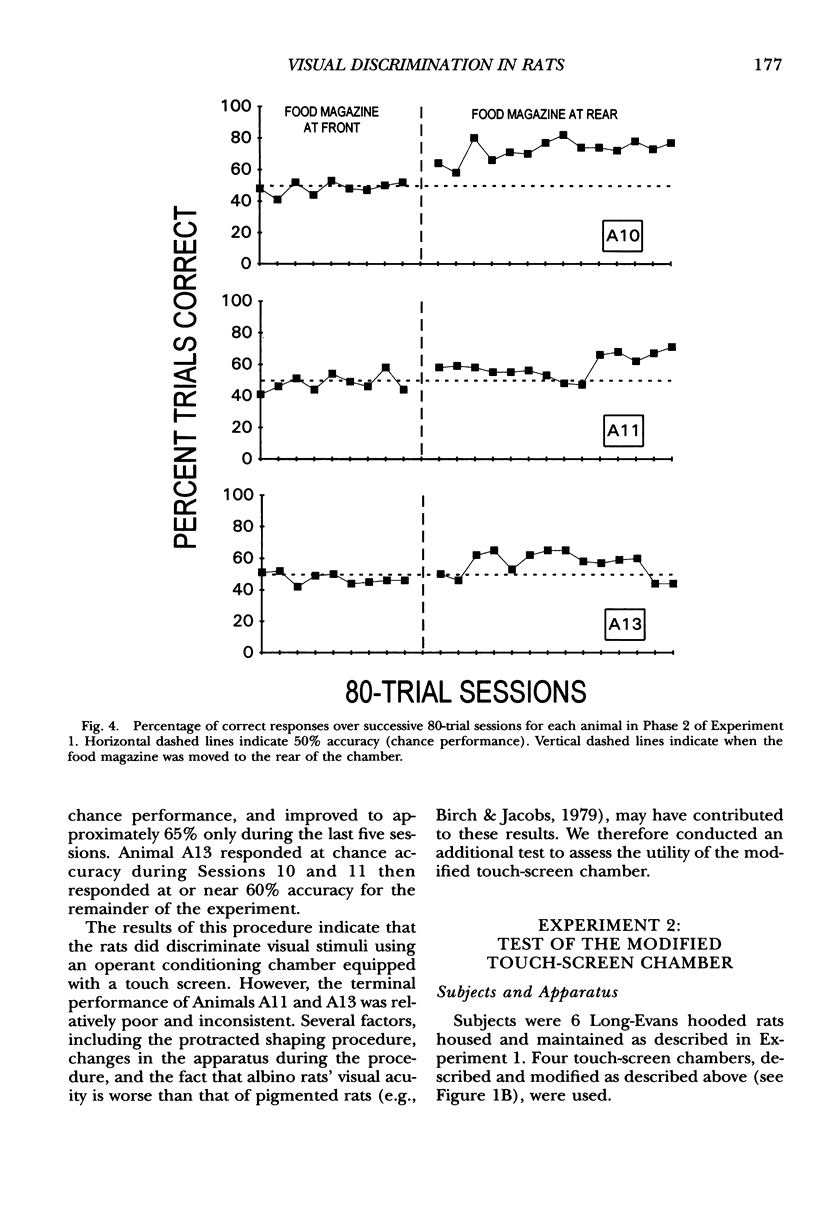
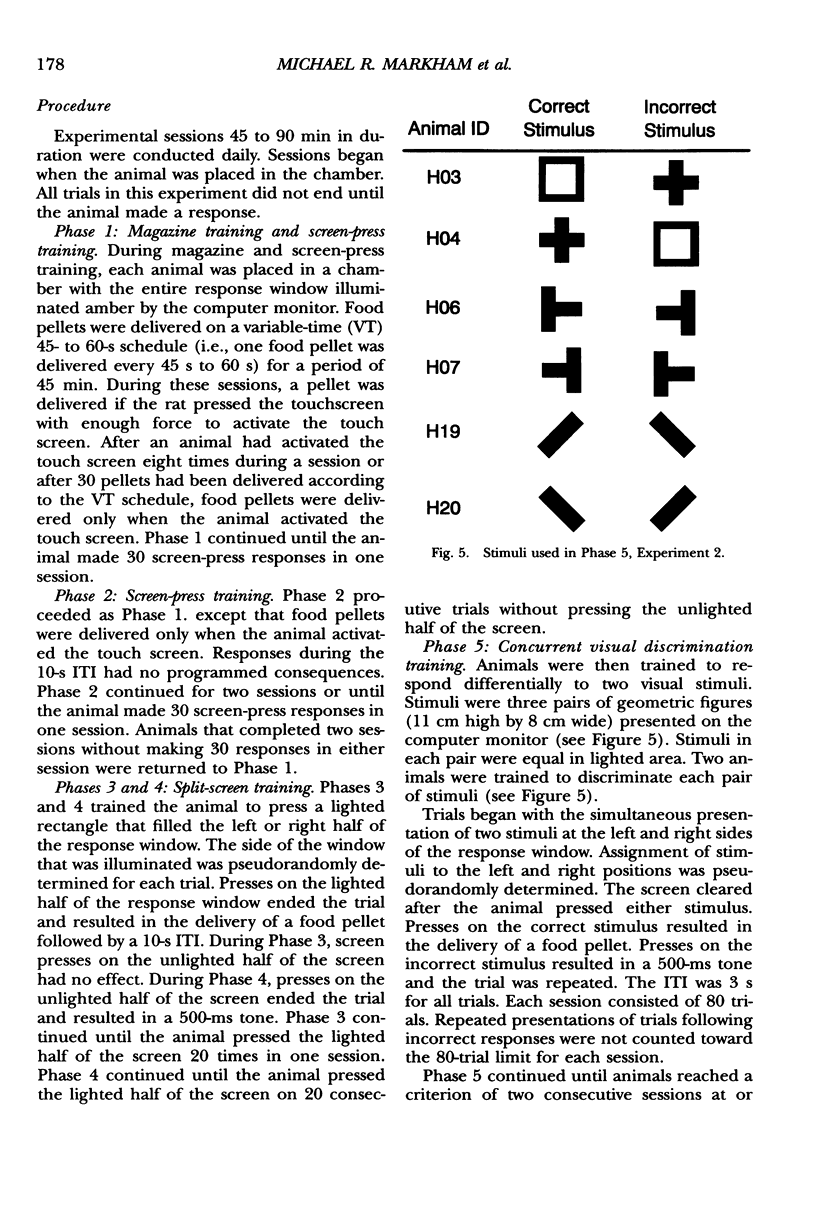
We describe an operant conditioning apparatus that uses computerized touch-screen technology and is designed for the versatile and highly controlled testing of rats in a potentially wide variety of behavioral paradigms. Although computer-controlled touch-screen systems have been developed for use with pigeons, monkeys, and humans, analogous technologies and methods have not yet been developed for rats. The development of a touch-screen system for rats could enhance the efficiency of behavioral research with rats, and may offer a unique tool for studying animal learning. In the first test of the utility of the apparatus, 3 Sprague-Dawley rats learned to activate the touch screen only after the touch-screen panel was made slightly movable. These animals then learned to discriminate visual stimuli presented on the computer monitor, but only after the food magazine and pellet dispenser were moved to the rear of the chamber opposite the stimulus display and response window. In a test of the utility of the modified apparatus, 6 Long-Evans rats learned to activate the touch screen and learned one of three different simple discriminations using computer-generated, visually presented stimuli. A basic method for training rats to activate the computer touch screen and for visual discrimination training is described. Results show that rats learned to activate the touch screen and discriminate visual stimuli presented on a computer monitor. Potential applications and advantages of the touch-screen-equipped rat operant conditioning chamber are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggleton J. P., Shaw C., Gaffan E. A. The performance of postencephalitic amnesic subjects on two behavioural tests of memory: concurrent discrimination learning and delayed matching-to-sample. Cortex. 1992 Sep;28(3):359–372. doi: 10.1016/s0010-9452(13)80146-3. [DOI] [PubMed] [Google Scholar]
- Bhatt R. S., Wright A. A. Concept learning by monkeys with video picture images and a touch screen. J Exp Anal Behav. 1992 Mar;57(2):219–225. doi: 10.1901/jeab.1992.57-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch D., Jacobs G. H. Spatial contrast sensitivity in albino and pigmented rats. Vision Res. 1979;19(8):933–937. doi: 10.1016/0042-6989(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Dean P. Are rats short-sighted? Effects of stimulus distance and size on visual detection. Q J Exp Psychol B. 1981 May;33B(2):69–76. doi: 10.1080/14640748108400813. [DOI] [PubMed] [Google Scholar]
- Green D. G., Powers M. K., Banks M. S. Depth of focus, eye size and visual acuity. Vision Res. 1980;20(10):827–835. doi: 10.1016/0042-6989(80)90063-2. [DOI] [PubMed] [Google Scholar]
- Lynch D. C., Green G. Development and crossmodal transfer of contextual control of emergent stimulus relations. J Exp Anal Behav. 1991 Jul;56(1):139–154. doi: 10.1901/jeab.1991.56-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. A., Gaffan D., Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993 Oct;13(10):4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


