Abstract
The effective treatment or cure of motoneuron disease will require understanding the disease processes that precede irreversible cell loss. To study these early stages, and to evaluate potential treatments in relevant animal models, requires a sensitive functional assay. To this end, we sought to determine whether the gait pattern of SOD1 transgenic mice changed prior to overt symptoms. Using a simplified video-based approach we compared the treadmill gait of C57BL/6J and B6.SOD1 transgenic mice at 8 and 10 weeks of age. B6.SOD1 mice had significantly longer stride and stance times than controls by 8 weeks. Consistent with disease progression, hindpaw measures of B6.SOD1 mice showed larger changes than front paws. Differences between control and B6.SOD1 mice increased at 10 weeks, but only because repeat testing caused habituation in control mice to a greater extent than in B6.SOD1 mice. Together the results demonstrate that simplified gait analysis is sensitive to early processes of motor system disease in mice.
Keywords: amyotrophic lateral sclerosis, gait dynamics, mice, SOD1, transgenic
Abbreviations: ALS, amyotrophic lateral sclerosis; B6, C57BL/6J; B6.SOD1, B6.Cg-Tg(SOD1-G93A); B6SJL, B6SJL-TgN(SOD1-G93A)1GUR; SOD1, superoxide dismutase 1
In amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases, functional deficits are preceded for an uncertain, but variable, interval by pathological changes. It is critical to understand these early disease processes so that optimal therapeutic targets, which not only arrest the disease but also prevent the development of irreversible functional deficits, can be identified.
Measurement and analysis of gait has been successfully applied to every common laboratory species and many others as well.5,24 Moreover, it has provided a detailed basic understanding of human and quadruped locomotion and is a now a common cross-species clinical tool that is sensitive to relatively minor changes associated with disease, injury, or rehabilitation.2,8,22,23 In its most sophisticated form, analysis of gait can include synchronized collection of ground reaction forces and kinematic and electromyographic data.13 The difficulty of scaling and applying these techniques to mice has limited their use despite a steady increase in studies of the murine motor system.15,16
A simple but related method that has been successfully used in mice is footprint analysis. This requires application of ink to the animal’s paws and then measurement of static gait parameters (e.g., stride length) from the resulting footprints.7,9,20 Such an approach is simple and reasonably sensitive but has significant practical limitations. We have extended it by using digital video capture of paw placements of mice during treadmill locomotion and then generating standard gait measures from the video images.
Using this method we describe the earliest functional deficits (8 weeks) that have been reported for SOD1 transgenic mice, at least to our knowledge. The onset of gait abnormalities correlates well with the reported timing of the loss of peripheral nerve connections in SOD110 and suggests that degeneration of distal axons may be among the earliest events with functional consequences. This simplified approach for generation of standard gait measures has wide applicability and is a useful complement to the powerful molecular and genetic tools that are available for the mouse.
MATERIALS AND METHODS
Mice.
The mice used in this study were inbred C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine), hereafter referred to as B6, and a new congenic strain of mice B6.Cg-Tg(SOD1-G93A)1Gur/J, hereafter referred to as B6.SOD1, carrying the G93A mutant form of the human SOD1 transgene.14 Strain background can have a powerful effect on phenotypic expression.11 The background strain of mice used for many studies of the human SOD1 mutation is a B6SJL mixed hybrid strain [B6SJL-TgN(SOD1-G93A)1GUR] maintained by crossing transgene-positive male mice with B6SJL F1 hybrid females. In order to facilitate further genetic studies and reduce phenotypic variability inherent in the random genetic background of the hybrid transgenics, we back-crossed mice carrying the mutant human SOD1 transgene with inbred B6 mice for more than ten generations to create the new B6.SOD1 congenic strain. C57BL/6J mice were chosen as the background strain because the entire B6 genome has been sequenced and is publicly available (www.en-sembl.org) and also because B6 is a common background strain for many transgenic and knockout mutations. The age of onset of overt symptoms was subjectively assessed4 and the age of death recorded for mice at each generation.
Mice were maintained in humidity- and temperature-controlled rooms with a 12:12 dark:light cycle. They were given an NIH-mouse/rat diet with 4% fat (5K54, PMI Feeds, Inc., St. Louis, MO) ad libitum with free access to water (HCl-acidified, pH 2.8–3.2). All procedures performed on the animals were reviewed and approved by our institutional animal care and use committee.
Gait Analysis Device.
A system was designed to allow video capture of the paws of the mice during treadmill locomotion (Fig. 1). Hardware consisted of a treadmill with a digital video camera (Basler A301fc, Basler, Inc., Exton, PA) mounted directly below a testing chamber that enclosed the mouse on the treadmill (Mouse Specifics, Inc., Boston, MA). The testing chamber was a rectangular plexiglass “corral” (20 × 4 × 16.5 cm) that ensured the mouse remained within view of the camera at all times. To obtain a distinct and detailed paw image the treadmill belt (13.8 × 500.7 cm) was clear and arranged such that only a single layer was between the camera lens (7.5 cm below) and the mouse paws (Fig. 1). Illumination of the testing enclosure was critical to obtaining an optimal contrast between the paws and body of the mouse. Contrast was optimized by placing a bright green backdrop on the ceiling of the test enclosure and mounting two tracks of six halogen bulbs (12V5W, Juno Lighting, Des Plaines, IL) at both edges 6 cm below the treadmill. The digital camera operated at 80 fps with a resolution of 658 × 494 pixels and the camera output was fed directly to a computer. Treadmill speed could be varied from 0 to 1.2 m/s.
FIGURE 1.
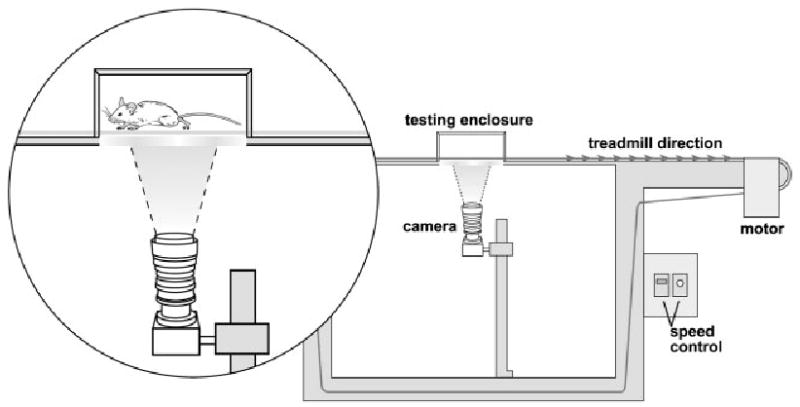
Arrangement of treadmill and digital video camera for capture of paw placement. The testing enclosure ensures that the animal remains within view of the camera. Treadmill speed is continuously variable with a maximum of 120 cm/s.
The software used in this study included two separate programs. Digital video-recording software was used to capture the raw video images and convert them to mpeg format (Video Savant Basic 4.0, IO Industries, Inc., London, Ontario, Canada). The mpeg file was then exported to a custom analysis program (Treadscan 1.0, Clever Sys, Inc., Reston, VA).
Data Collection.
Camera settings, lighting, and treadmill speed were set prior to introduction of the mouse. The animal was placed into the testing chamber and the treadmill was turned on where it could be seen live on the computer screen. As soon as the mouse was walking somewhat consistently (i.e., straight line, fixed position relative to the camera) the animal was recorded for a fixed period (12 s, see below) after which the treadmill was stopped and the mouse was removed from the testing chamber.
A step cycle or stride consists of two consecutive contacts of a given paw. Preliminary work showed that collection of five or six strides was sufficient; fewer than this increased variability, whereas additional strides beyond this number did not greatly reduce variability. To ensure that we always had a sufficient number of strides, we captured 958 frames at 80 fps (~12 s). From this video clip, we selected a portion of the video in which the animal consistently stayed in the same position relative to the camera (i.e., maintained the selected treadmill speed) for at least five strides per paw (mean, 11 ± 3.6; minimum = 5, maximum = 20).
The speeds we used (17 cm/s and 23 cm/s) were selected because we sought to validate a protocol that would be suitable for our high-throughput screening program (www.jax.org/nmf). Using these speeds, sufficient data could be collected in 30– 60 s. The speeds were selected based on preliminary data collected on ten wild-type B6 mice. The slowest speed at which consistent behavior on the treadmill was obtained within a reasonable time for B6 mice was 13 cm/s and the fastest was 25 cm/s. Although data can be collected outside of this speed range, obtaining a consistent pattern is more difficult. This is particularly true for slower speeds; even at 17 cm/s it was more difficult to get the mice to walk with a consistent pattern.
Gait Parameters.
Analysis software determines when individual paws are in contact with the treadmill and then uses this determination to derive standard gait parameters. Stride time is the time between two initial paw contacts with the treadmill for the same paw. Stance time is the portion of the stride time that the paw is in contact with the treadmill, and the swing time is the portion of the stride time that the paw is not in contact with the treadmill surface. Representative values for each parameter were calculated using the average of consecutive strides (5–20) for each of the four paws. For group comparisons, the right and left paws were averaged to give representative values for the front and rear paws for each animal.
Experimental Design and Data Analysis.
We compared data collected from two groups of animals: inbred C57BL/6J (n = 10; 3 females, 7 males) and transgenic B6.Cg-Tg(SOD1-G93A)1Gur/J mice (n = 10; 6 females, 4 males). Data were collected for all four paws for each group of mice in four testing sessions. At 8 weeks of age, mice were tested at a slow speed (17 cm/s) and then rested on the treadmill for at least 5 min before testing at the fast speed (23 cm/s). Two weeks later, both groups (10-weeks-old) repeated testing at both speeds. A four-way repeated-measures analysis of variance (ANOVA) was performed with group, paw, age, and speed as factors. Tukey’s honest significant difference (HSD) test was used to compare individual means. Student’s t-test was used for comparison of data in complementary studies. P ≤ 0.05 was used as a limit for declaring statistical significance.
RESULTS
Genetic Background Increases Survival.
In our laboratory the B6.SOD1 congenic mice, at backcross generations N10–N14, survived significantly longer than mice carrying the transgene on the typical B6SJL hybrid background [50% survival: 157.1 ± 9.3 days (n = 45) vs. 128.9 ± 9.1 days (n = 67), respectively; log-rank chi-square, P < 0.0001] (Fig. 2). The increased survival was evident after only two generations of backcrossing (50% survival: 143 ± 7.6 days; n = 13). The age of onset for B6.SOD1 congenics at N10 was 142.3 ± 10.6 days (observable tremors in at least one hindlimb while suspending mice by the tail4) and the mean time from onset to death was 16.5 ± 9.3 days. We found no gender differences in survival for either the B6SJL F1 generation (P = 0.15) or the B6 N10+ generations (P = 0.43) in contrast to that observed by others in mice carrying a low-copy G93A transgene.25
FIGURE 2.
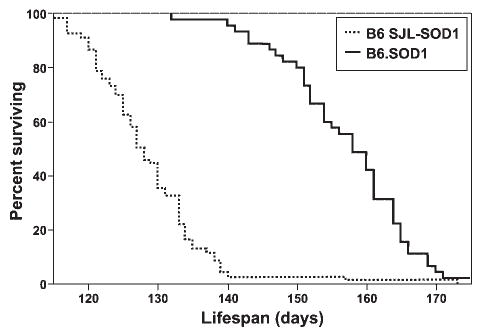
Kaplan–Meier survival comparison of the B6.SOD1 mice after ten backcross generations and hybrid B6SJL-SOD1(G93A) mice.
Gait Dynamics: Validation.
To validate the use of this simplified approach to measuring standard gait parameters, we first examined the data for control mice to confirm that they showed the expected gait adjustments when treadmill speed was changed.5 Comparison of values captured at 17 cm/s and 23 cm/s for C57BL/6J mice (Fig. 3, also see Fig. 4) revealed a significant speed-related decrease in each of the three basic parameters (P < 0.001, P < 0.01, and P < 0.05 for stride, stance, and swing time, respectively) at both test ages. The increase in treadmill speed also significantly reduced stance/stride time ratio and commensurately increased the swing/stride time ratio (not shown). Finally, consistent with previous work,5 there was no difference between front and rear paws for stride time of B6 controls (averaged across age and speed) but the mean stance time of front paws was shorter (P < 0.003; 174.0 ± 27 vs. 184 ± 23 for front and rear, respectively) and swing time was commensurately, although not significantly, longer (P = 0.06; 120 ± 21 vs. 111 ± 20 for front and rear, respectively).
FIGURE 3.
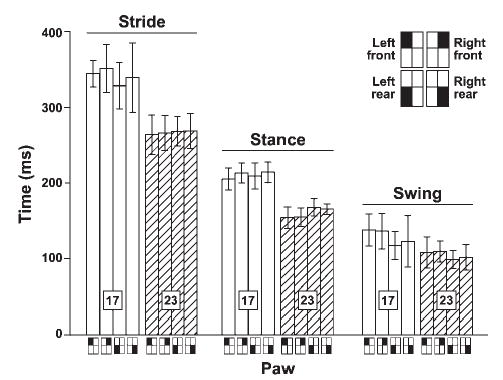
Plot shows the three basic gait parameters (stride, stance, and swing time; mean ± SD) for all four limbs at two different speeds (open columns: 17 cm/s; hatched columns: 23 cm/s) for C57BL/6J control mice at 8 weeks of age (n = 10). These data show the expected speed-related decrease in each parameter.
FIGURE 4.
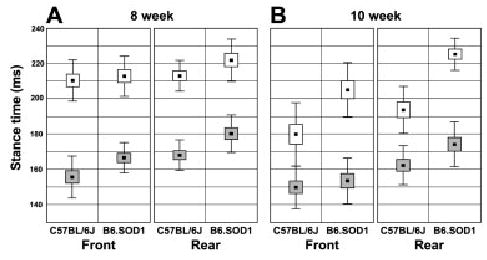
Box-and-whisker plot comparisons of mean stance times of front and rear paws for B6.SOD1 and B6 mice at both test ages and speeds. Error bars are ± 1 standard deviation and boxes are ± 1 standard error. Open boxes: 17 cm/s; shaded boxes: 23 cm/s.
Early Gait Differences in B6.SOD1 Congenic Mice.
Our next step was to determine whether B6.SOD1 congenic mice could be distinguished from B6 controls prior to the overt signs of disease that become apparent at approximately 20 weeks of age. Overall, compared to controls, B6.SOD1 mice walked with significantly longer stride and stance times [295 ± 40 vs. 314 ± 40, F(1,9) = 9.1, P = 0.01 for stance; 179 ± 26 vs. 192 ± 28 F(1,9) = 18, P = 0.002 for stride], but the small increase in swing time did not reach statistical significance (116 ± 21 vs. 121 ± 20). Consistent with previous work,5 stance time was a sensitive index of gait differences between B6.SOD1 mice and controls.
Post hoc comparison of group means for stance time at both ages showed that B6.SOD1 mice were different from controls by 8 weeks of age (P < 0.05). However, the group difference at 8 weeks was revealed most clearly by the faster treadmill speed. For example, increased stance time at 8 weeks was apparent at the slower speed (open symbols in Fig. 4A, 17 cm/s), particularly for the rear paws, but a significant difference between control and transgenics only emerged, for both front and rear paws, when the mice were required to walk faster (shaded symbols in Fig. 4A, 23 cm/s; P = 0.05 and P < 0.02 for front and rear, respectively). Overall, the difference in mean stance time between control and B6.SOD1 mice (averaged across both speeds and ages) was relatively greater for the rear paws as revealed by the significant two-way interaction [genotype × paw interaction, F(1,9) = 5.9, P = 0.04] displayed in Figure 5A. Closer examination shows that this effect was more marked at 10 weeks of age when the difference between front and rear paws for B6.SOD1 was nearly double that of controls (Fig. 5B). Similar interaction effects for stance/stride and swing/stride time ratios (data not shown) also demonstrated this effect; in the front paws, both ratios were nearly identical between genotypes (P = 0.86 for stance/stride, P = 0.76 for swing/stride), whereas for the rear paws the stance/stride ratio was different (P = 0.05) and the swing/stride time ratio approached statistical significance (P = 0.06).
FIGURE 5.
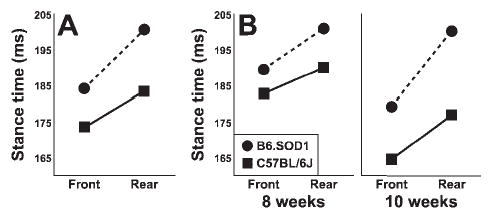
(A) Two-way interaction plot [genotype × paw; F(1,9) = 5.92, P < 0.05] shows mean stance time averaged across both speeds and ages. The difference between B6.SOD1 and B6 is greater for the rear paws than for the front paws. (B) Differential effect on front and rear paws is evident at both ages but is more marked at 10 weeks.
Disease Progression and Habituation.
We next determined whether gait measures were sensitive to disease progression in B6.SOD1 mice over a 2-week period. Analysis of variance did reveal a significant main effect of test age on stance time [F(1,9) = 19.1, P = 0.002]. Overall stance times were shorter at 10 than 8 weeks of age (180 ± 27 vs. 191 ± 28). This effect opposed that observed between genotypes (i.e., stride and stance times of B6 > B6.SOD1). Thus, it is not surprising that post hoc comparison of B6.SOD1 means at 8 and 10 weeks revealed no statistical difference (P > 0.2), indicating that B6.SOD1 gait measures remained relatively stable over this period (Fig. 4). In contrast, stance time of B6 control mice was significantly shorter at 10 weeks than at 8 weeks (P = 0.001; also stride time, not shown).
Gait dynamics in freely walking mice were found to be stable with age beyond postnatal day 24,6 suggesting that gait changes did not accompany age-related changes in size and weight. To evaluate whether this was true for mice walking on a treadmill, we compared gait parameters of separate naive groups (n = 4) of 8- and 10-week-old mice collected on the same day. Despite significant differences in weight (t-test, P < 0.05) comparison of naive groups showed no effect of age (t-test, P > 0.5 for all comparisons) on stride, stance, or swing times (averaged across paws: 343 ± 38 vs. 342 ± 47, 227 ± 21 vs. 216 ± 26, and 121 ± 24 vs. 126 ± 36 for 8-week vs. 10-week stride, stance, and swing time, respectively). Furthermore, when these same mice were retested on the following day, stride and stance time decreased significantly (t-test, P < 0.02 for all comparisons), but the swing time did not reach statistical significance (averaged across paws and age: 342 ± 41 vs. 309 ± 28, 218 ± 21 vs. 191 ± 21, and 124 ± 30 vs. 118 ± 18 for test 1 vs. test 2 stride, stance, and swing time, respectively). These data confirm that normal mice habituated to treadmill walking with repeat testing.
However, the retest effect was complex as it varied not only between experimental groups, but also with speed, as demonstrated by a significant three-way interaction for stance time [genotype × age × speed; F(1,9) = 5.2, P < 0.05].
This interaction effect demonstrates that the control and transgenic animals differed in the extent of habituation. This effect is seen most clearly by close examination of the data collected at slower speed (open symbols in Fig. 4). At 17 cm/s, B6 stance time of control mice was shorter at 10 weeks than at 8 weeks for both the front and rear paws (20–30 ms). However, the same comparison for B6.SOD1 mice showed a perceptible increase for the rear paws and only a relatively small (~10 ms) decrease for the front paws. Interestingly, when speed was increased (23 cm/s; filled symbols in Fig. 4), habituation of B6 stance time was less (~10 ms) than at the slower speed, but at that point the habituation was evident, although subtle, for both the front and rear paws of B6.SOD1 mice.
Gait Measures as a Screening Tool.
We analyzed the gait parameters for 129 mutagenized B6 mice (n = 59 females, n = 70 males). The data were collected for 8–9-week-old mice at 23 cm/s (Table 1). The coefficients of variation ranged from ~7–20%, with swing time of the front paws being the most variable. Although male and female data distributions largely overlap, this large sample revealed gender differences: mean stride, stance, and swing times of male mice were longer than for females (Table 1). These data establish baseline distributions to guide identification of potential deviants/outliers within mutagenized pedigrees but also are generally useful to laboratories that might choose to collect similar data on C57BL/6J mice.
Table 1.
Gait parameters of mutagenized C57BL/6J mice at 23 cm/s.
| Males (n = 70)
|
Females (n = 59)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Front
|
Rear
|
Front
|
Rear
|
|||||||||
| Mean ± SD | −95% | 95% | Mean ± SD | −95% | 95% | Mean ± SD | −95% | 95% | Mean ± SD | −95% | 95% | |
| Stride (ms) | ||||||||||||
| Right | 289 ± 35 | 281 | 297 | 283 ± 24 | 277 | 289 | 273 ± 31 | 265 | 281 | 268 ± 25 | 262 | 275 |
| Left | 287 ± 32 | 279 | 294 | 283 ± 26 | 277 | 289 | 268 ± 35 | 259 | 277 | 273 ± 27 | 266 | 280 |
| Stance (ms) | ||||||||||||
| Right | 172 ± 17 | 168 | 176 | 177 ± 14 | 174 | 180 | 162 ± 17 | 157 | 166 | 169 ± 15 | 165 | 173 |
| Left | 169 ± 15 | 166 | 173 | 178 ± 14 | 175 | 181 | 162 ± 15 | 158 | 166 | 171 ± 14 | 167 | 175 |
| Swing (ms) | ||||||||||||
| Right | 117 ± 28 | 110 | 124 | 106 ± 18 | 102 | 110 | 111 ± 27 | 104 | 118 | 100 ± 15 | 96 | 104 |
| Left | 117 ± 26 | 110 | 123 | 105 ± 19 | 100 | 110 | 106 ± 27 | 99 | 113 | 102 ± 18 | 97 | 106 |
| Stance/stride | ||||||||||||
| Right | 0.61 ± 0.04 | 0.60 | 0.62 | 0.63 ± 0.03 | 0.62 | 0.64 | 0.60 ± 0.05 | 0.59 | 0.61 | 0.63 ± 0.03 | 0.62 | 0.64 |
| Left | 0.60 ± 0.05 | 0.59 | 0.61 | 0.64 ± 0.03 | 0.63 | 0.64 | 0.61 ± 0.03 | 0.61 | 0.62 | 0.63 ± 0.03 | 0.62 | 0.64 |
| Swing/stride | ||||||||||||
| Right | 0.39 ± 0.04 | 0.38 | 0.40 | 0.37 ± 0.03 | 0.36 | 0.38 | 0.39 ± 0.05 | 0.38 | 0.4 | 0.37 ± 0.03 | 0.36 | 0.38 |
| Left | 0.39 ± 0.04 | 0.38 | 0.40 | 0.36 ± 0.03 | 0.36 | 0.37 | 0.38 ± 0.04 | 0.37 | 0.39 | 0.37 ± 0.03 | 0.36 | 0.38 |
Mean, standard deviation, and the lower (−95%) and upper (+95%) limits of the P = 0.05 confidence intervals are shown. Mice were third-generation progeny of founder mice treated with ethyl-nitro sourea (ENU) to create random point mutations. All mice were recessive for a subset of the ENU-induced mutations in the founder with any specific mutation represented in approximately one in eight of the offspring. None had overt phenotypes at the time of testing. Note the slightly longer mean stride, stance, and swing times of the male mice.
DISCUSSION
Influence of Genetic Background.
This study has provided data on a new inbred strain of mice carrying the mutant G93A human SOD1 transgene. The expression of the transgene on the B6 background significantly delayed disease onset (Fig. 2), but the time from onset to death was comparable to that of the hybrid mice. These data suggest the presence of B6 genes that delay the appearance of overt symptoms. Once that “threshold” is reached, however, the decline in B6.SOD1 mice is as rapid as in the mixed B6SJL mice carrying the SOD1 transgene. The difference between B6.SOD1 mice and mixed B6SJL transgenic mice may also be partly explained by the fact that an unknown percentage of the mixed hybrid transgenics are homozygous for a dysferlin mutation in the SJL strain that results in limb-girdle muscular dystrophy.1
Preliminary data from ongoing work suggest that the pathology in B6.SOD1 mice is the same as in hybrid SOD1 mice but that its appearance is delayed (K. Seburn, unpublished observations). Transgene copynumber between the two transgenic strains was the same, but experiments to measure transgene expression are not yet complete, so it is possible that the delayed onset is due to a genetic background–specific reduction in expression. An alternative explanation is provided by reports that exercise can increase lifespan or delay onset of symptoms.19,26 C57BL/6J mice are among the most active strains (Mouse Phenome Database, www.jax.org), whereas SJL mice are among the least active, with B6/SJL hybrids, as predicted, intermediate between the two (K. Seburn, unpublished data).
The increased survival of B6.SOD1 mice underlines the importance of genetic background. As one example, making the human transgene congenic on the B6 background produced an effect as powerful as a 31-day delay in onset achieved by viral delivery of insulin-like growth factor-1 in mixed B6SJL SOD1 transgenics.17 The use of the hybrid mice in such studies leaves open the possibility that genetic differences between litters could obscure or enhance treatment effects.
Gait Parameters Provide Sensitive Measures.
Our simplified approach to gait analysis provided data comparable to those reported in previous studies using more sophisticated methods.5,6,21 The approach is rapid (~15 min per mouse) and sufficiently sensitive to detect early functional changes in progressive motor neuron disease. Abnormalities in standard gait parameters of B6.SOD1 mice are evident by 8 weeks of age, whereas the appearance of an observable hindlimb tremor when animals are suspended by the tail4 occurs, on average, at ~20 weeks.
A comparison of the stride and stance times for B6.SOD1 transgenic mice at 8 and 10 weeks showed no significant change over the 2-week period of the study, which suggests that disease progression caused no detectable changes in gait over this period or that changes were too subtle to be detected. However, the opposing effect unexpectedly introduced by habituation to repeat testing (discussed below), tempers this conclusion. When tested at 17 cm/s at 10 weeks, the rear paw stance time of the B6.SOD1 mice showed a perceptible increase, despite the opposing effect of habituation that was clear from the significant decrease observed in the rear paw stance time of control mice (as well as in forepaw stance time of both groups) (Fig. 4). Differences between 8- and 10-week B6.SOD1 measures may have become evident if data were collected on separate groups of naive mice.
Taken together, these results demonstrate that the accumulated effects of mutant transgene expression modified gait patterns as early as 8 weeks.
Neither the timing nor the nature of the precipitating events that eventually lead to overt functional deficits, motoneuron loss, and ultimately death in the presence of the mutant SOD1 G93A transgene, are known. However, our results demonstrate that the deleterious effects of the mutant transgene precede overt signs of the disease (and probably motor neuron loss10). Indeed, it seems likely that pathological processes have been underway for some time and that gait abnormalities could be evident prior to 8 weeks.
The mechanism responsible for the observed gait changes in B6.SOD1 transgenics requires further investigation, but the timing of their appearance corresponds well with the timing of early changes at the level of the motor unit or neuromuscular junction (6–7 weeks) previously reported in transgenic mice of mixed hybrid backgrounds.10,12,18 Fischer and colleagues10 confirmed that loss of synaptic connections at the neuromuscular junction noted in their study preceded any loss of motoneurons. As such, basic gait parameters provide a useful assay for studying these early changes and evaluating potential therapies that could be implemented prior to irreversible cell loss.
Mice Habituate to Treadmill Walking.
The habituation response we observed was unexpected given the brief exposures to the treadmill. This was not an effect of age-related variation in weight and size, and we confirmed the habituation response in a separate group of normal mice studied for this report. In addition, we have recently observed the effect again while examining alcohol-induced changes in gait parameters of 8-week-old B6 mice. A remaining alternative explanation is that mice systematically exceeded the prescribed treadmill speed with repeated exposure. This could happen because mice sometimes use a “run and coast” strategy, particularly at the slower speed. We avoided portions of the video where this occurred; nonetheless, to exclude this possibility, we calculated and compared stride frequency (2.2 and 2.9 Hz; 17 and 23 cm/s, respectively) and found no difference for mice tested at 8 and 10 weeks of age [F(1,9) = 1.9, P = 0.18].
Habituation to treadmill walking has, to our knowledge, not been described specifically for mice but the phenomenon is well documented. It is known that quadrupeds2,27 and humans modify their normal gait dynamics when required to walk on a treadmill.3 In humans this habituation is both rapid and ongoing. For example, humans show significant changes in gait kinematics within 10 s of beginning to walk on a treadmill3 and, within a session, subjects take more than 10 min to develop a consistent pattern.28 Moreover, subjects continue to show an initial, although accelerated, habituation response across multiple sessions.29
Mice in our study were given no prior training to become accustomed to walking on the treadmill. Such training might reduce the habituation effect but could also introduce additional confounding effects related to increased activity. In any case, this phenomenon needs to be considered in experimental design.
Finally, our large-scale screening project has collected a large data set describing murine gait (Table 1). These data revealed a gender difference in basic gait parameters that, to our knowledge, has not been previously observed. These differences could be due to gender differences in weight, size, or gait mechanics. Previous work showing gait parameters to be stable with age (and therefore weight),6 and our demonstration that 8- and 10-week-old mice were not different, both support the belief that the gender difference is not related to size differences. However, these comparisons are imperfect (e.g., freely moving mice vs. treadmill, small sample sizes, strain differences) and additional study is required to resolve this issue definitively (e.g., weight-matched male vs. female comparison). Nonetheless, gender differences seem to contribute to variability and should also be considered in experimental design.
Acknowledgments
This study was supported by funding from the ALS Association (K. Seburn) and NIH Grant NS41215 (W. N. Frankel). The authors thank Dr. Verity Letts and Dr. Leah Rae Donahue for their critical reading of the manuscript.
References
- 1.Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, et al. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet. 1999;23:141–142. doi: 10.1038/13770. [DOI] [PubMed] [Google Scholar]
- 2.Buchner DM, Cress ME, Esselman PC, Margherita AJ, de Lateur BJ, Campbell AJ, et al. Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 1996;51:M297–302. doi: 10.1093/gerona/51a.6.m297. [DOI] [PubMed] [Google Scholar]
- 3.Charteris J, Taves C. The process of habituation to treadmill walking: a kinematic analysis. Percept Mot Skills. 1978;47:659–666. doi: 10.2466/pms.1978.47.2.659. [DOI] [PubMed] [Google Scholar]
- 4.Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, et al. Age-dependent penetrance of disease in a trans-genic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- 5.Clarke KA, Still J. Gait analysis in the mouse. Physiol Behav. 1999;66:723–729. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- 6.Clarke KA, Still J. Development and consistency of gait in the mouse. Physiol Behav. 2001;73:159–164. doi: 10.1016/s0031-9384(01)00444-9. [DOI] [PubMed] [Google Scholar]
- 7.Costa AC, Walsh K, Davisson MT. Motor dysfunction in a mouse model for Down syndrome. Physiol Behav. 1999;68:211–220. doi: 10.1016/s0031-9384(99)00178-x. [DOI] [PubMed] [Google Scholar]
- 8.Engsberg JR, Lauryssen C, Ross SA, Hollman JH, Walker D, Wippold FJ., II Spasticity, strength, and gait changes after surgery for cervical spondylotic myelopathy: a case report. Spine. 2003;28:E136–139. doi: 10.1097/01.BRS.0000051878.74535.F7. [DOI] [PubMed] [Google Scholar]
- 9.Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepi-androsterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75:391–400. doi: 10.1002/jnr.10821. [DOI] [PubMed] [Google Scholar]
- 10.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Frankel WN. Mouse strain backgrounds: more than black and white. Neuron. 1998;20:183. doi: 10.1016/s0896-6273(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 12.Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gronley JK, Perry J. Gait analysis techniques. Rancho Los Amigos Hospital gait laboratory Phys Ther. 1984;64:1831–1838. doi: 10.1093/ptj/64.12.1831. [DOI] [PubMed] [Google Scholar]
- 14.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, Rockenstein E, Masliah E. Transgenic models of alpha-synuclein pathology: past, present, and future. Ann NY Acad Sci. 2003;991:171–188. [PubMed] [Google Scholar]
- 16.Jankowsky JL, Savonenko A, Schilling G, Wang J, Xu G, Borchelt DR. Transgenic mouse models of neurodegenerative disease: opportunities for therapeutic development. Curr Neurol Neurosci Rep. 2002;2:457–464. doi: 10.1007/s11910-002-0073-7. [DOI] [PubMed] [Google Scholar]
- 17.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 18.Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport. 1996;7:1427–1431. doi: 10.1097/00001756-199605310-00021. [DOI] [PubMed] [Google Scholar]
- 19.Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53:804–807. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- 20.Klapdor K, Dulfer BG, Hammann A, Van der Staay FJ. A low-cost method to analyse footprint patterns. J Neurosci Methods. 1997;75:49–54. doi: 10.1016/s0165-0270(97)00042-3. [DOI] [PubMed] [Google Scholar]
- 21.Leblond H, L’Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neurosci. 2003;23:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsolais GS, Dvorak G, Conzemius MG. Effects of postoperative rehabilitation on limb function after cranial cruciate ligament repair in dogs. J Am Vet Med Assoc. 2002;220:1325–1330. doi: 10.2460/javma.2002.220.1325. [DOI] [PubMed] [Google Scholar]
- 23.Mohr KJ, Kvitne RS, Pink MM, Fideler B, Perry J. Electromyography of the quadriceps in patellofemoral pain with patellar subluxation. Clin Orthop. 2003:261–271. doi: 10.1097/01.blo.0000093918.26658.6a. [DOI] [PubMed] [Google Scholar]
- 24.Muybridge E. Human and animal locomotion. New York: Dover Publications; 1979.
- 25.Trieu VN, Uckun FM. Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem Biophys Res Commun. 1999;258:685–688. doi: 10.1006/bbrc.1999.0577. [DOI] [PubMed] [Google Scholar]
- 26.Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 27.Vilensky JA, Patrick MC. Inter and intratrial variation in cat locomotor behavior. Physiol Behav. 1984;33:733–743. doi: 10.1016/0031-9384(84)90040-4. [DOI] [PubMed] [Google Scholar]
- 28.Wall JC, Charteris J. The process of habituation to treadmill walking at different velocities. Ergonomics. 1980;23:425–435. doi: 10.1080/00140138008924758. [DOI] [PubMed] [Google Scholar]
- 29.Wall JC, Charteris J. A kinematic study of long-term habituation to treadmill walking. Ergonomics. 1981;24:531–542. doi: 10.1080/00140138108924874. [DOI] [PubMed] [Google Scholar]


