Abstract
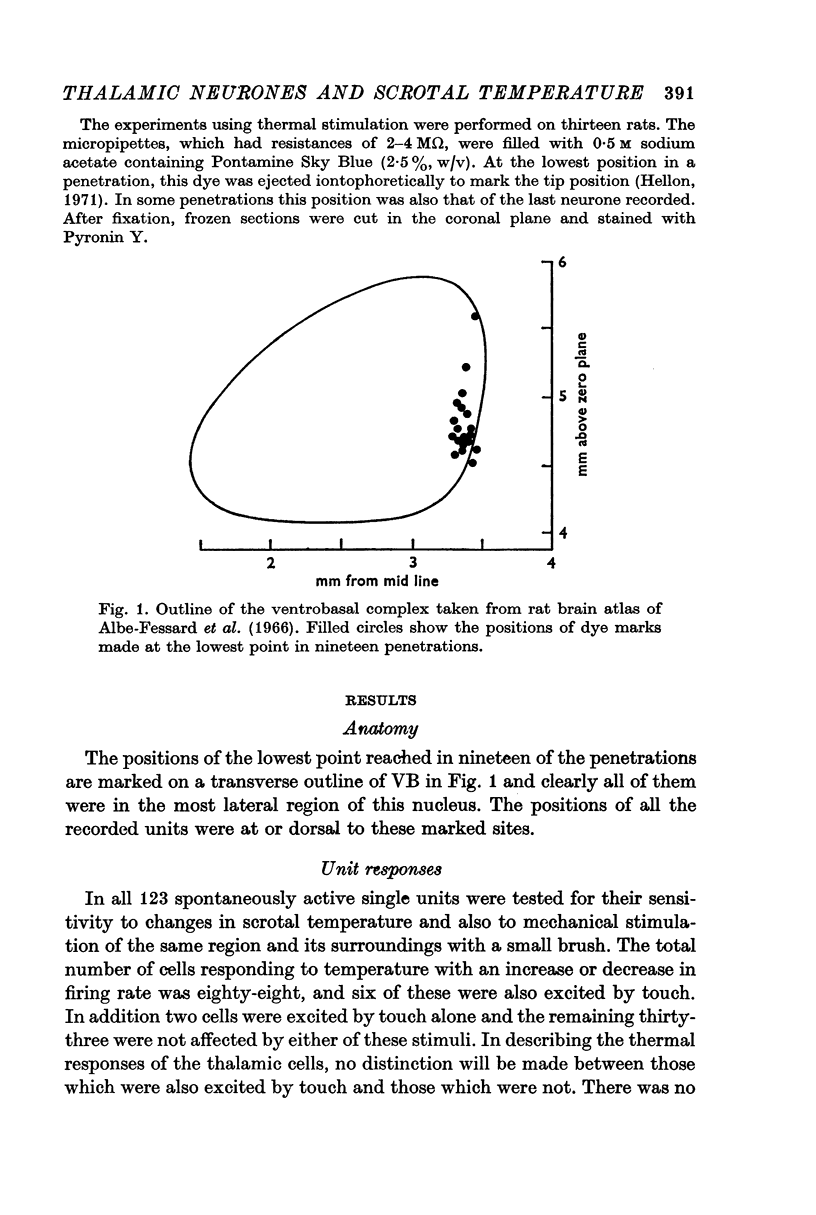
1. In rats the scrotal temperature was raised or lowered with a water-perfused thermode while micro-electrode recordings were made of unit activity in the ventrobasal complex of the thalamus. The electrodes were aimed at the region where evoked responses had been found by electrical stimulation of the scrotum. Recording sites were marked by iontophoresis of dye from the micro-electrode.
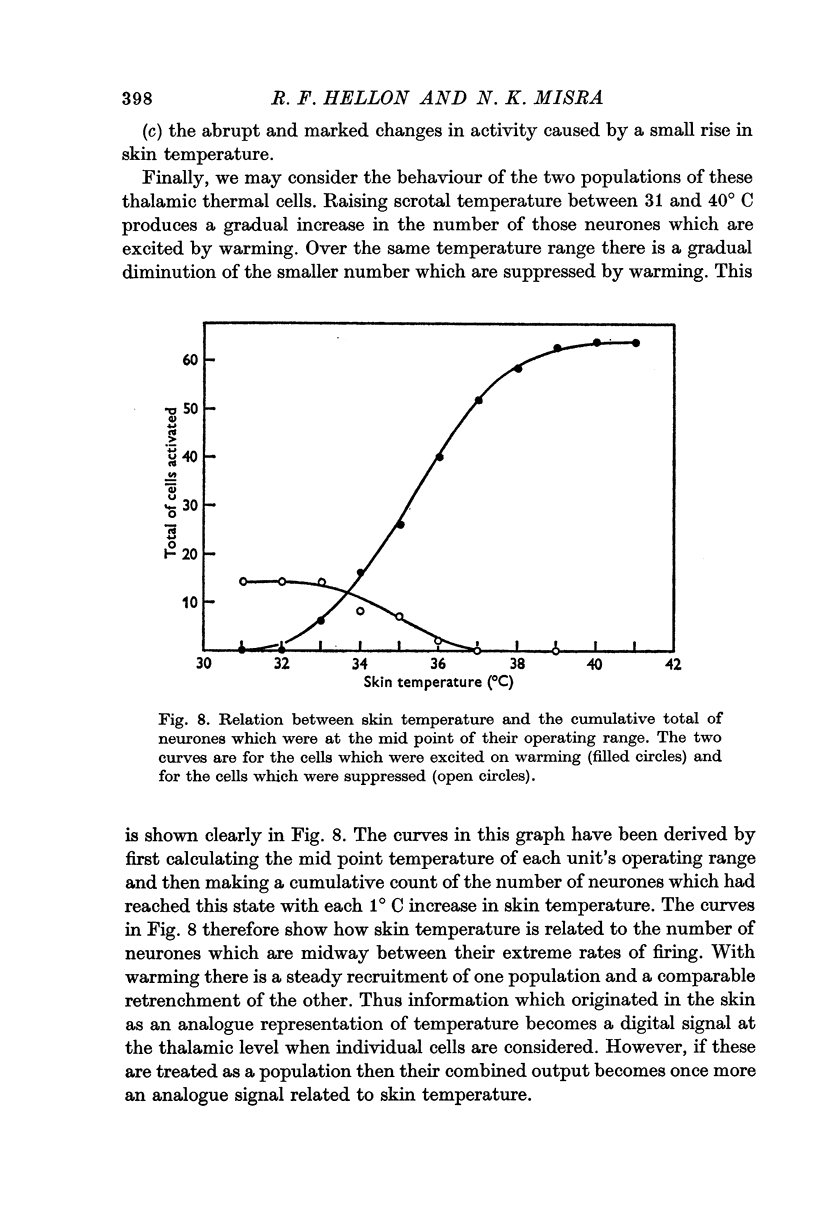
2. Changes in firing rate of thalamic neurones were only found in the scrotal temperature range of 31-40° C. Within this range, 72% of the 123 cells tested were excited or suppressed by skin warming. At temperatures above or below this range, activity was not affected. Most of the cells responded just to temperature and only 7% were also excited by touch.
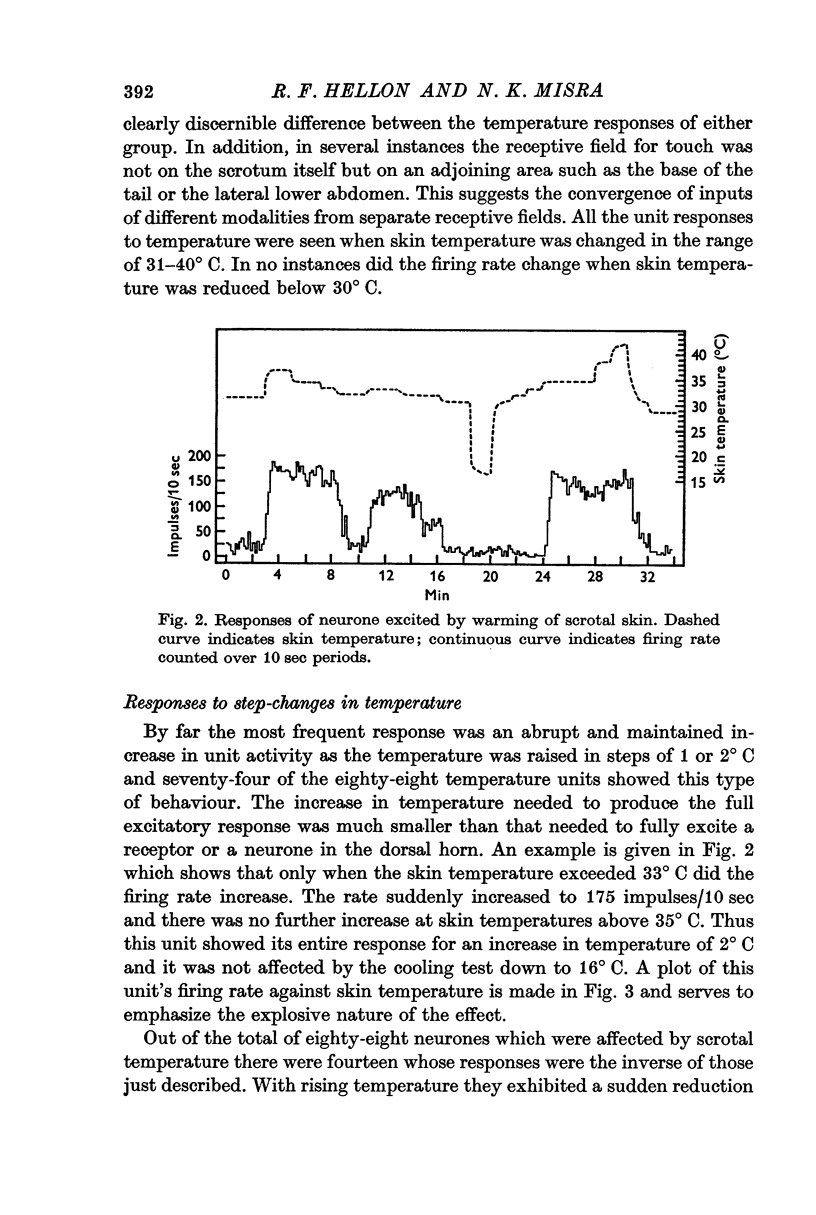
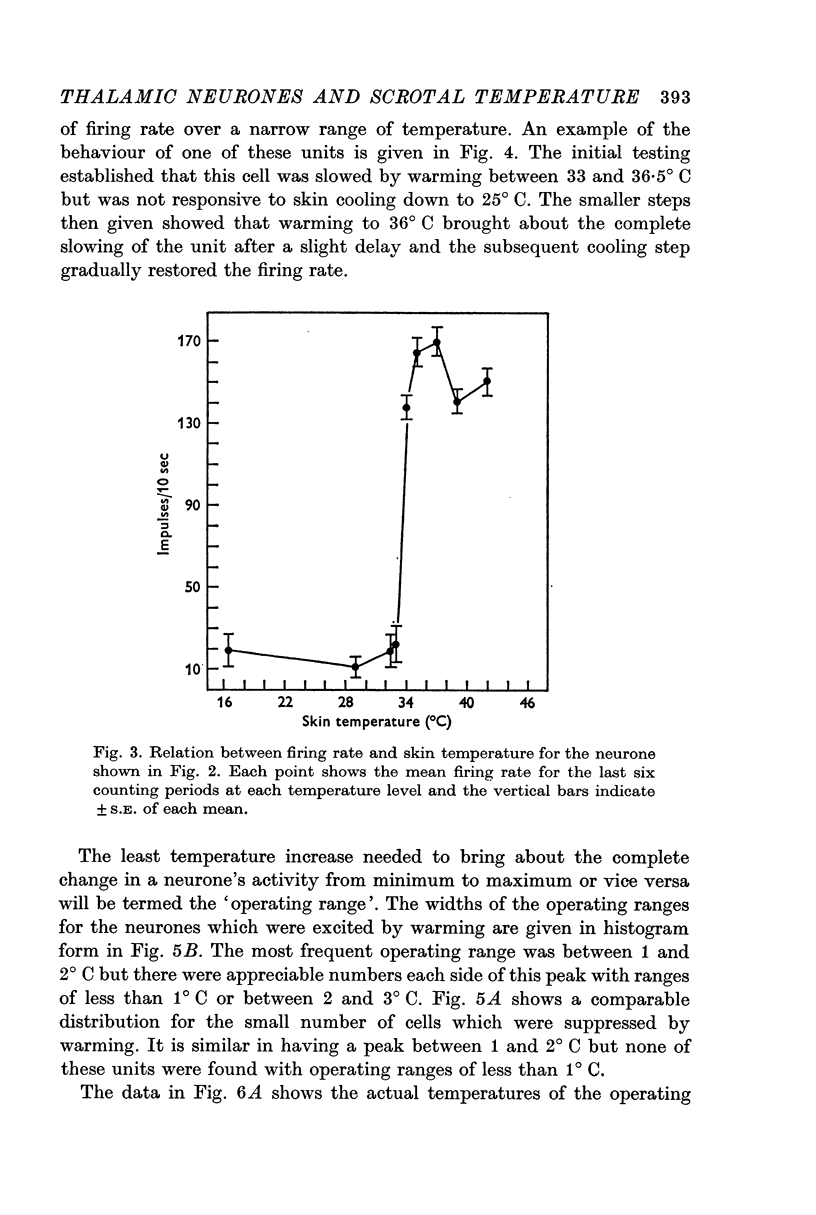
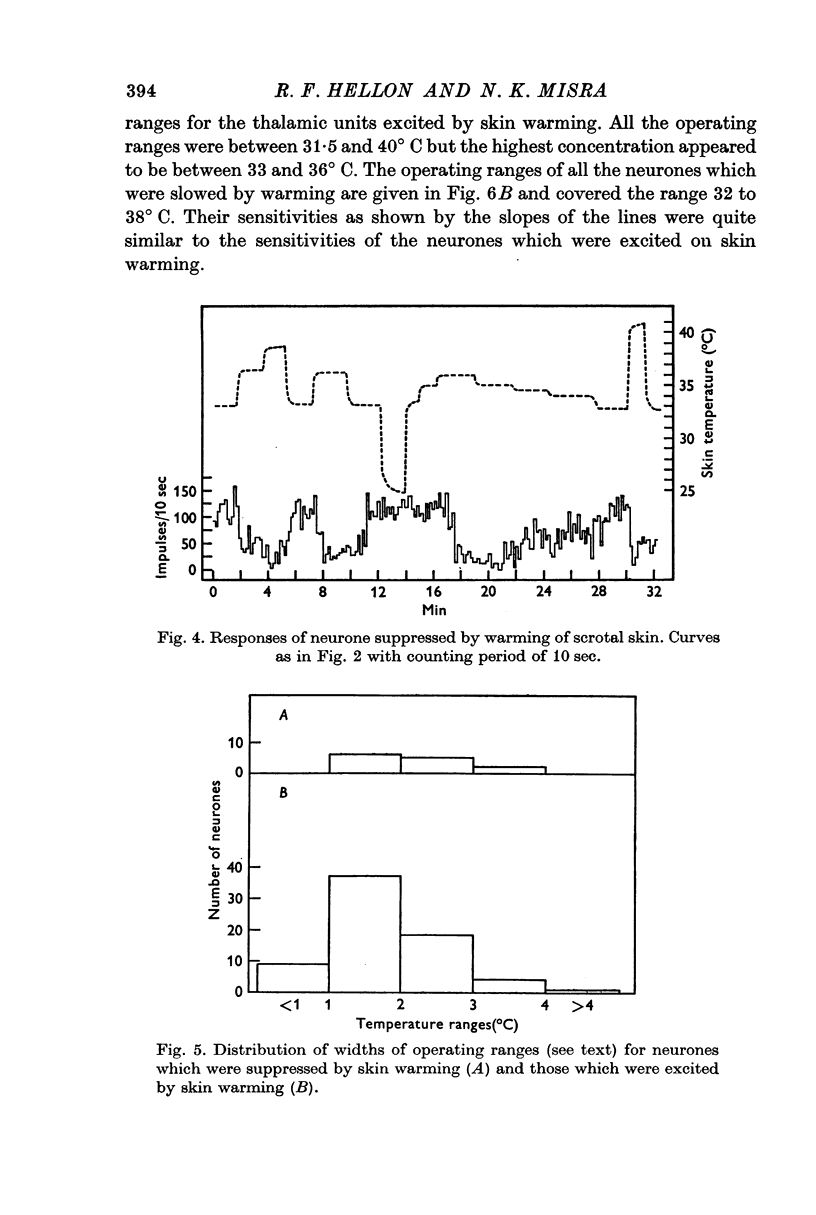
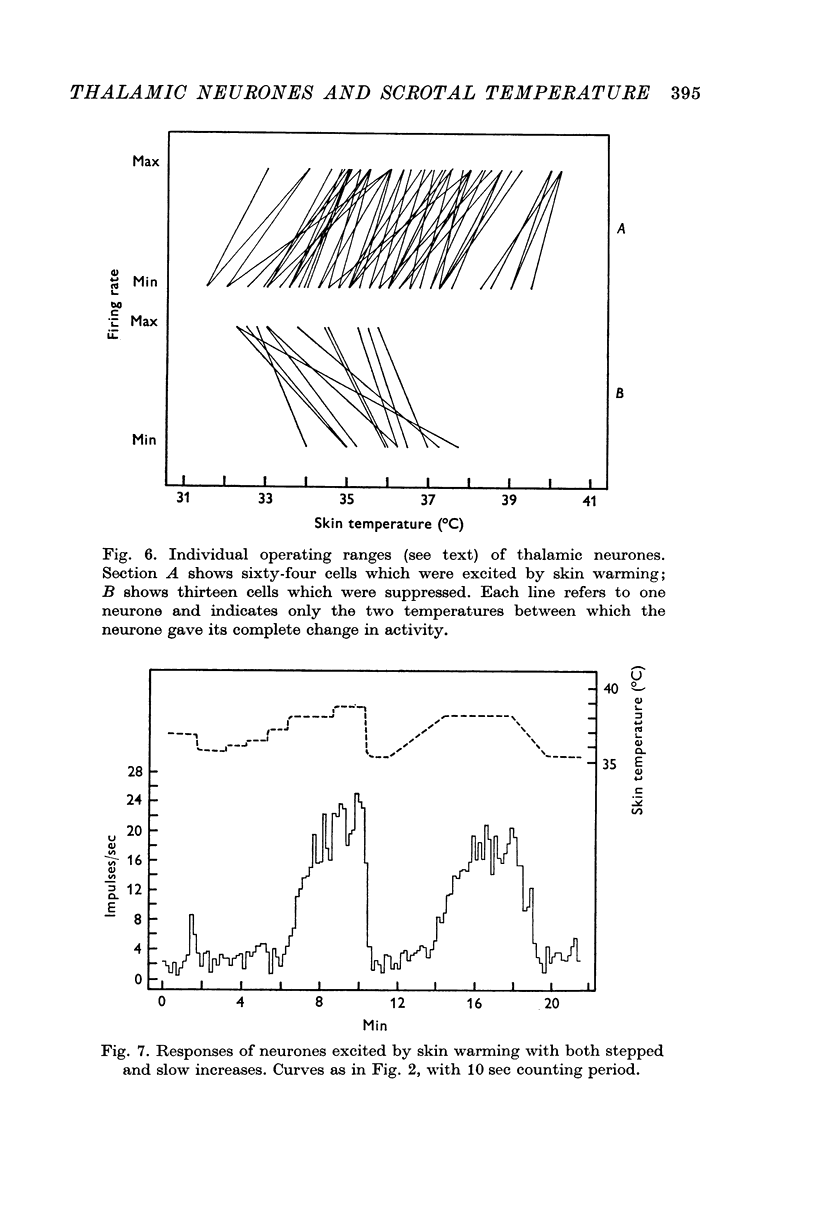
3. Raising temperature in the range 31-40° C caused 82% of the thermally responding cells to increase their firing rate and 18% to decrease their rate. Individual neurones showed a sudden and maintained change in their activity for scrotal temperature increases of only 2, 1 or even 0·5° C. Mean firing rates changed by factors of about 8 or more with these temperature increases and further warming did not change the rate. These step-like changes in firing rate were found at different points over the whole skin temperature range of 31-40° C, but most were between 33 and 38° C.
4. For a given neurone the step-like change in activity occurred once its critical temperature was reached, irrespective of whether this was achieved by a step increase of skin temperature over 1-2 sec or by a slow ramp increase lasting several minutes.
5. It is not possible to say whether the skin warm receptors, cold receptors or both were responsible for these thalamic responses, but the results do show that incoming thermal information is considerably processed when it reaches the thalamic level.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton H., Forbes D. J., Benjamin R. M. Thalamic neurons responsive to temperature changes of glabrous hand and foot skin in squirrel monkey. Brain Res. 1970 Dec 1;24(2):179–190. doi: 10.1016/0006-8993(70)90099-5. [DOI] [PubMed] [Google Scholar]
- Davidson N. The projection of afferent pathways on the thalamus of the rat. J Comp Neurol. 1965 Jun;124(3):377–390. doi: 10.1002/cne.901240308. [DOI] [PubMed] [Google Scholar]
- Duclaux R., Kenshalo D. R. The temperature sensitivity of the type I slowly adapting mechanoreceptors in cats and monkeys. J Physiol. 1972 Aug;224(3):647–664. doi: 10.1113/jphysiol.1972.sp009917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMERS R. ORGANIZATION OF THE FIRST AND THE SECOND SOMESTHETIC REGIONS (SI AND SII) IN THE RAT THALAMUS. J Comp Neurol. 1965 Apr;124:215–227. doi: 10.1002/cne.901240207. [DOI] [PubMed] [Google Scholar]
- Emmers R. Separate relays of tactile, pressure, thermal, and gustatory modalities in the cat thalamus. Proc Soc Exp Biol Med. 1966 Feb;121(2):527–531. doi: 10.3181/00379727-121-30821. [DOI] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the dorsal horn of the rat responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):375–388. doi: 10.1113/jphysiol.1973.sp010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Thalamic neurones responding to scrotal skin temperature in rats. J Physiol. 1972 Sep;225(2):41P–42P. [PubMed] [Google Scholar]
- Hellon R. F. The marking of electrode tip positions in nervous tissue. J Physiol. 1971;214 (Suppl):12P–12P. [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDGREN S. Thalamic neurones responding to cooling of the cat's tongue. Acta Physiol Scand. 1960 Mar 18;48:255–267. doi: 10.1111/j.1748-1716.1960.tb01860.x. [DOI] [PubMed] [Google Scholar]
- Lister W. C., Woodget L. L. Precision stereotaxic equipment. J Physiol. 1972 Apr;222(2):130P–132P. [PubMed] [Google Scholar]
- Martin H. F., 3rd, Manning J. W. Thalamic 'warming' and 'cooling' units responding to cutaneous stimulation. Brain Res. 1971 Apr 2;27(2):377–381. doi: 10.1016/0006-8993(71)90265-4. [DOI] [PubMed] [Google Scholar]
- Poulos D. A., Benjamin R. M. Response of thalamic neurons to thermal stimulation of the tongue. J Neurophysiol. 1968 Jan;31(1):28–43. doi: 10.1152/jn.1968.31.1.28. [DOI] [PubMed] [Google Scholar]
- Poulos D. A., Lende R. A. Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. I. Steady-state response. J Neurophysiol. 1970 Jul;33(4):508–517. doi: 10.1152/jn.1970.33.4.508. [DOI] [PubMed] [Google Scholar]
- Poulos D. A., Lende R. A. Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. II. Temperature change response. J Neurophysiol. 1970 Jul;33(4):518–526. doi: 10.1152/jn.1970.33.4.518. [DOI] [PubMed] [Google Scholar]


