Abstract
1. In rats the scrotal temperature was raised or lowered with a water-perfused thermode while micro-electrode recordings were made of unit activity in the somatosensory (SI) cortex. The electrodes were inserted in the area where the largest evoked potentials had been found from electrical stimulation of the scrotum.
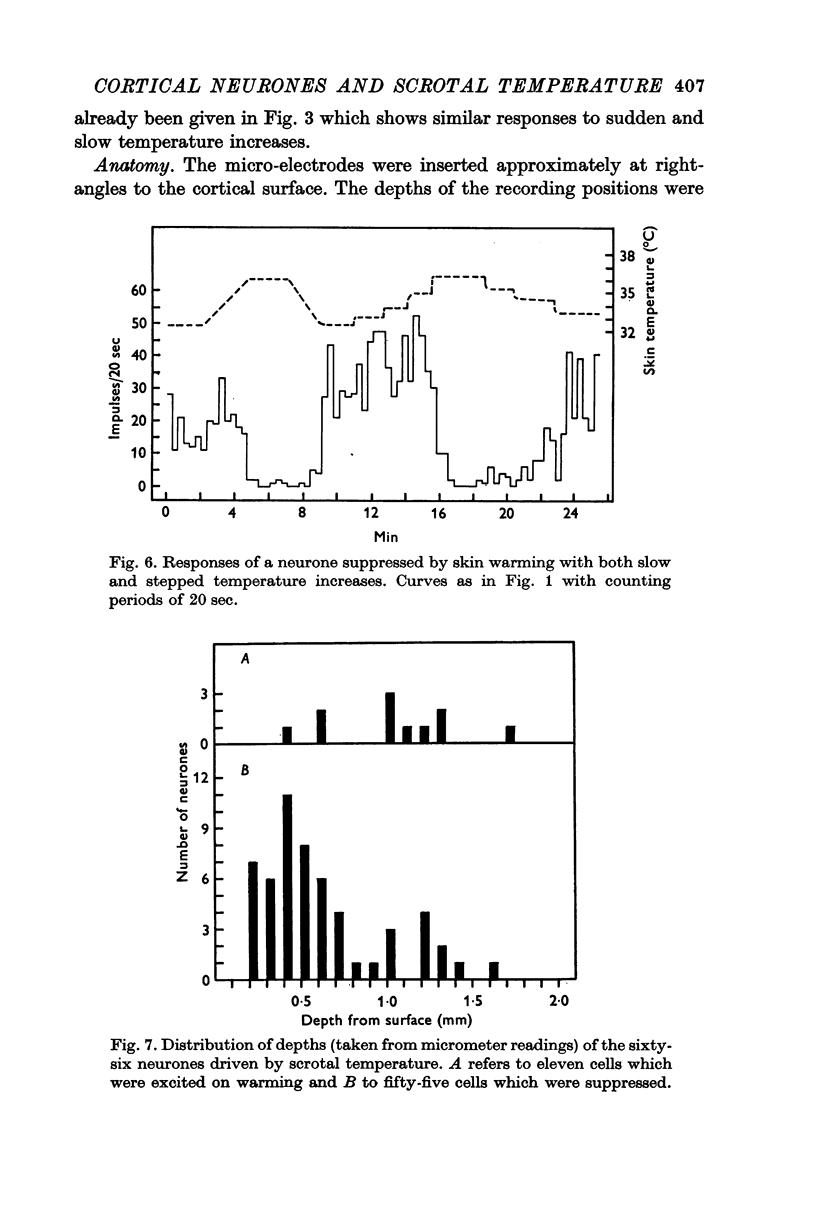
2. Changes in firing rate of cortical neurones were found only in the scrotal temperature range of 32-41° C. Within this range 40% of all the cells tested were excited or suppressed by skin warming. At temperatures above or below this range, activity was not affected. Most of the cells responded just to temperature and only 14% were also excited by touch.
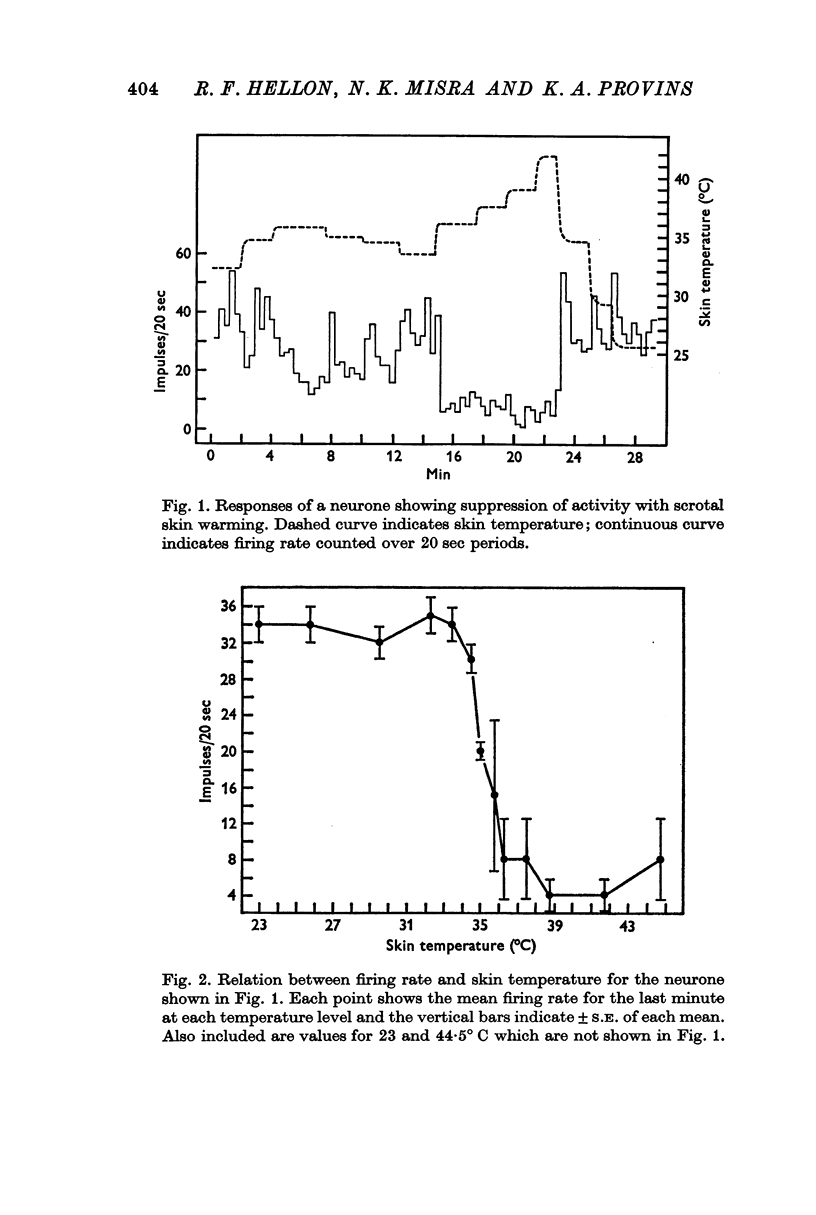
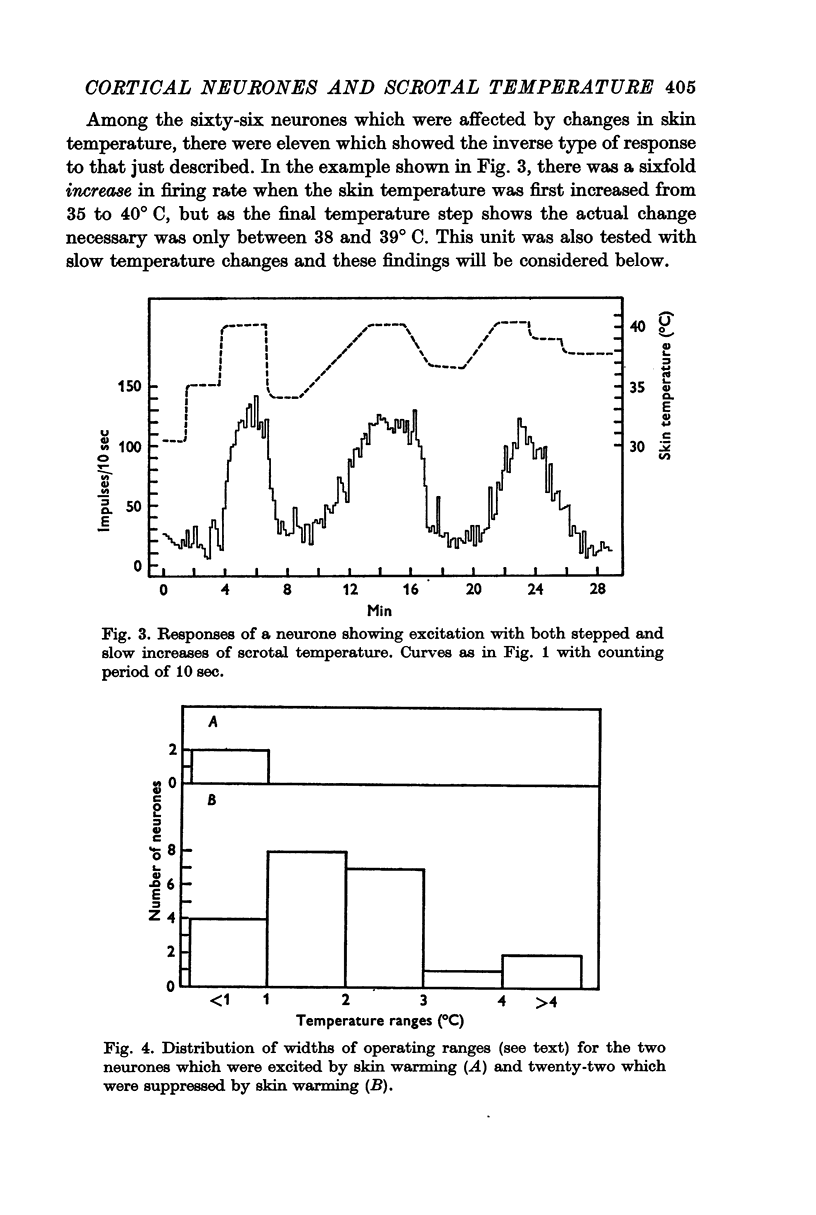
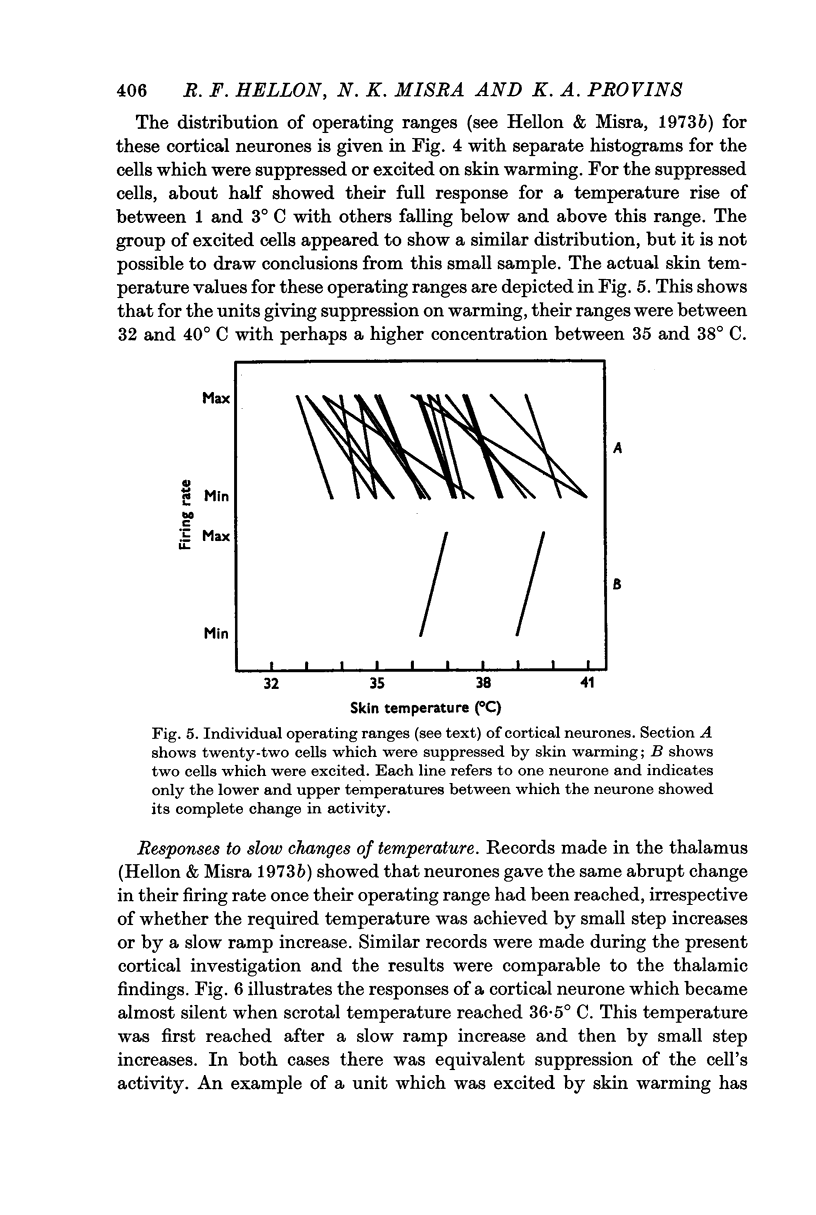
3. Raising temperature in the range 33-41° C caused 83% of the thermally responding cells to decrease their firing rate and 17% to increase their rate. Individual neurones showed a sudden and maintained change in their activity for scrotal temperature increases of only 2, 1 or even 0·5° C. Mean firing rates changed several-fold with these temperature increases and further warming did not change the rate. These step-like changes in firing rate were found at different points over the whole skin temperature range of 33-41° C but most were between 35 and 39° C.
4. For a given neurone the step-like change in activity occurred once its critical temperature was reached, irrespective of whether this was achieved by a step increase of skin temperature over 1-2 sec, or by a slow ramp increase lasting several minutes.
5. The results are very similar to those found in the thalamus (preceding paper), but the proportions of cortical cells which were excited or suppressed on skin warming were the reverse of the proportions seen in the thalamus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins P., Lacy D. Studies on the structure and function of the mammalian testis. II. Cytological and histochemical observations on the testis of the rat after a single exposure to heat applied for different lengths of time. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1026):17–38. doi: 10.1098/rspb.1969.0009. [DOI] [PubMed] [Google Scholar]
- Hales J. R., Hutchinson J. C. Metabolic, respiratory and vasomotor responses to heating the scrotum of the ram. J Physiol. 1971 Jan;212(2):353–375. doi: 10.1113/jphysiol.1971.sp009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the dorsal horn of the rat responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):375–388. doi: 10.1113/jphysiol.1973.sp010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the ventrobasal complex of the rat thalamus responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):389–399. doi: 10.1113/jphysiol.1973.sp010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Provins K. A. Unit responses in the somatosensory cerebral cortex of the rat following temperature changes in scrotal skin. J Physiol. 1972 Apr;222(2):151P–152P. [PubMed] [Google Scholar]
- Ingram D. L., Legge K. F. The influence of deep body and skin temperatures on thermoregulatory responses to heating of the scrotum in pigs. J Physiol. 1972 Jul;224(2):477–487. doi: 10.1113/jphysiol.1972.sp009906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisman N. R., Zimmerman I. D. Cortical unit responses to temperature stimulation of the skin. Brain Res. 1971 Jan 8;25(1):184–187. doi: 10.1016/0006-8993(71)90578-6. [DOI] [PubMed] [Google Scholar]
- LANDGREN S. Cortical reception of cold impulses from the tongue of the cat. Acta Physiol Scand. 1957 Oct 10;40(2-3):202–209. doi: 10.1111/j.1748-1716.1957.tb01489.x. [DOI] [PubMed] [Google Scholar]
- LANDGREN S. Thalamic neurones responding to cooling of the cat's tongue. Acta Physiol Scand. 1960 Mar 18;48:255–267. doi: 10.1111/j.1748-1716.1960.tb01860.x. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- POWELL T. P., MOUNTCASTLE V. B. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull Johns Hopkins Hosp. 1959 Sep;105:133–162. [PubMed] [Google Scholar]
- Poulos D. A., Benjamin R. M. Response of thalamic neurons to thermal stimulation of the tongue. J Neurophysiol. 1968 Jan;31(1):28–43. doi: 10.1152/jn.1968.31.1.28. [DOI] [PubMed] [Google Scholar]
- WAITES G. M. The effect of heating the scrotum of the ram on respiration and body temperature. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:314–323. doi: 10.1113/expphysiol.1962.sp001615. [DOI] [PubMed] [Google Scholar]


