Fig. 1.
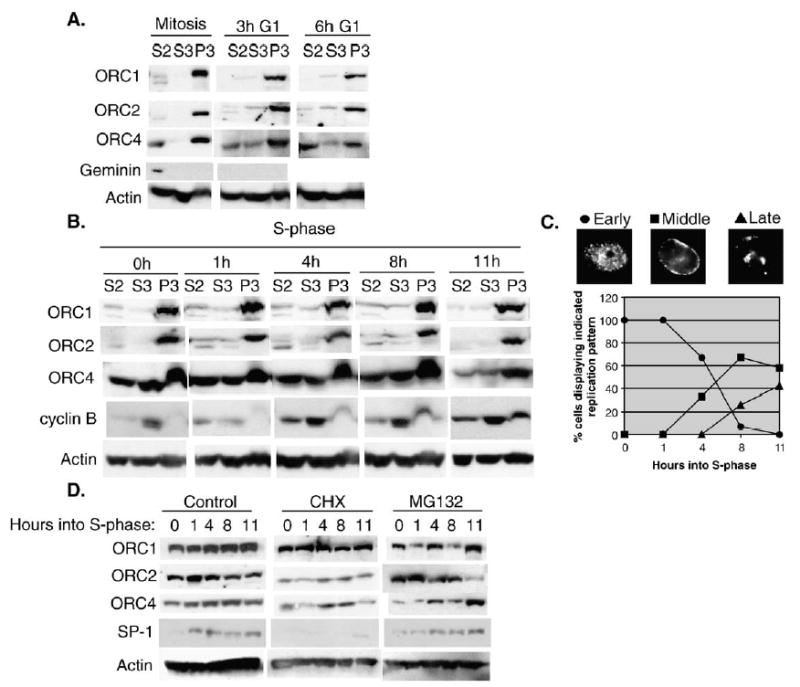
ORC is stable and chromatin-associated throughout the cell-cycle in CHO cells. (A) CHO cells were synchronized in mitosis with a brief (4 h) nocodazole block and collected and fractionated at the indicated timepoints as described in Materials and methods. Cell extracts were subjected to electrophoresis on 8% SDS-PAGE gels and immunoblotted with anti-ORC1, 2 and 4. Anti-geminin and anti-actin were used as controls for mitotic release and protein levels. The S2 and S3 fractions represent soluble protein fractions and the P3 fraction represents the chromatin-enriched fraction. (B) CHO cells were synchronized in S-phase and cells fractionated at 0, 1, 4, 8 or 11 h as described in Materials and methods. As a control for S-phase progression, extracts were immunoblotted with anti-cyclin B1. Anti-actin was used as a loading control. Similar results were obtained in 4 independent experiments (3 times with aphidicolin and once with mimosine arrested cells). The results of two of these experiments were additionally confirmed using an anti-HsORC1 antibody (gift of J. Mendez and B. Stillman, not shown). (C) To monitor S-phase progression, aliquots of cells from (B) were pulse labeled with BrdU for 30 min, fixed and stained with anti-BrdU. The percentage of cells displaying the indicated replication pattern (^ Early, ▪ Middle, ▴ Late) was scored. (D) CHO cells synchronized in S-phase as in panel (B) were released into media containing: DMSO (control), 50 μg/ml cycloheximide or 25 μM MG132 for 0, 1, 4, 8 and 11 h. Whole cell extracts were prepared and immunoblotted for ORC1, 2, 4, SP1 and actin. Similar results were obtained in two independent experiments.
