Fig. 3.
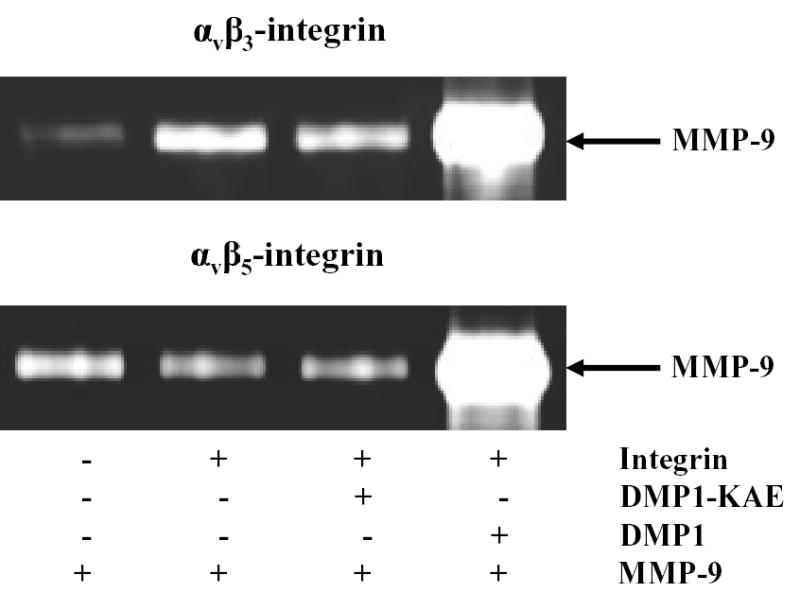
Pull-down experiments showing bridging of matrix metalloproteinase 9 (MMP-9) to αvβ3 integrin and αvβ5 integrin by dentin matrix protein 1 (DMP1). The αvβ3 or αvβ5 integrins were first bound to their respective monoclonal antibodies previously attached to beads by the manufacturer. After washing, the beads were incubated with buffer alone or buffer containing 500 nM DMP1-KAE or 500 nM DMP1, washed, and subsequently treated with recombinant proMMP-9. The washed samples were then electrophoresed on 10% zymogram gelatin gels and examined by Coomassie blue staining after digestion conditions were performed. Beads alone have low background level of MMP-9 binding (Lane 1). Note that the addition of DMP1 (Lane 4) but not DMP1-KAE (lane 3) enabled proMMP-9 to be pulled down with both sets of integrin-bound beads. Control levels of proMMP-9 were observed without addition of DMP1 (Lane 2).
