Abstract
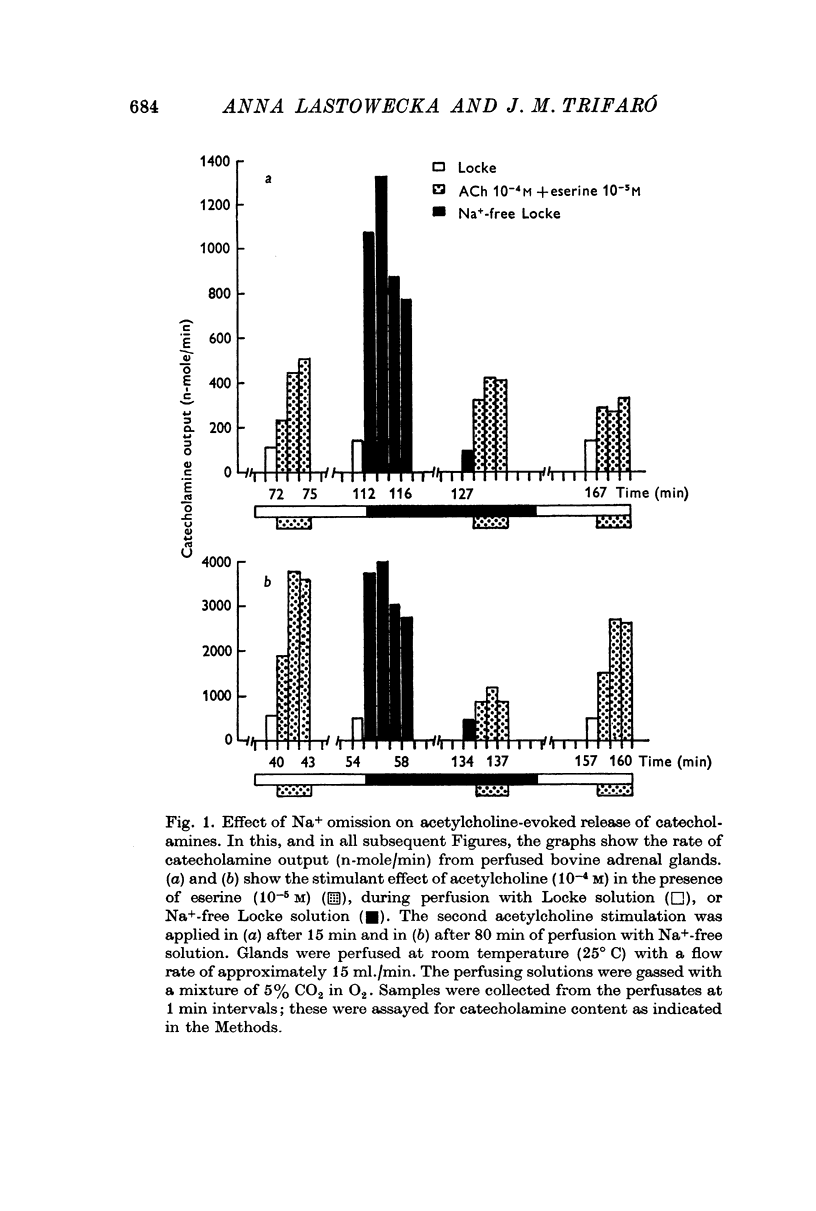
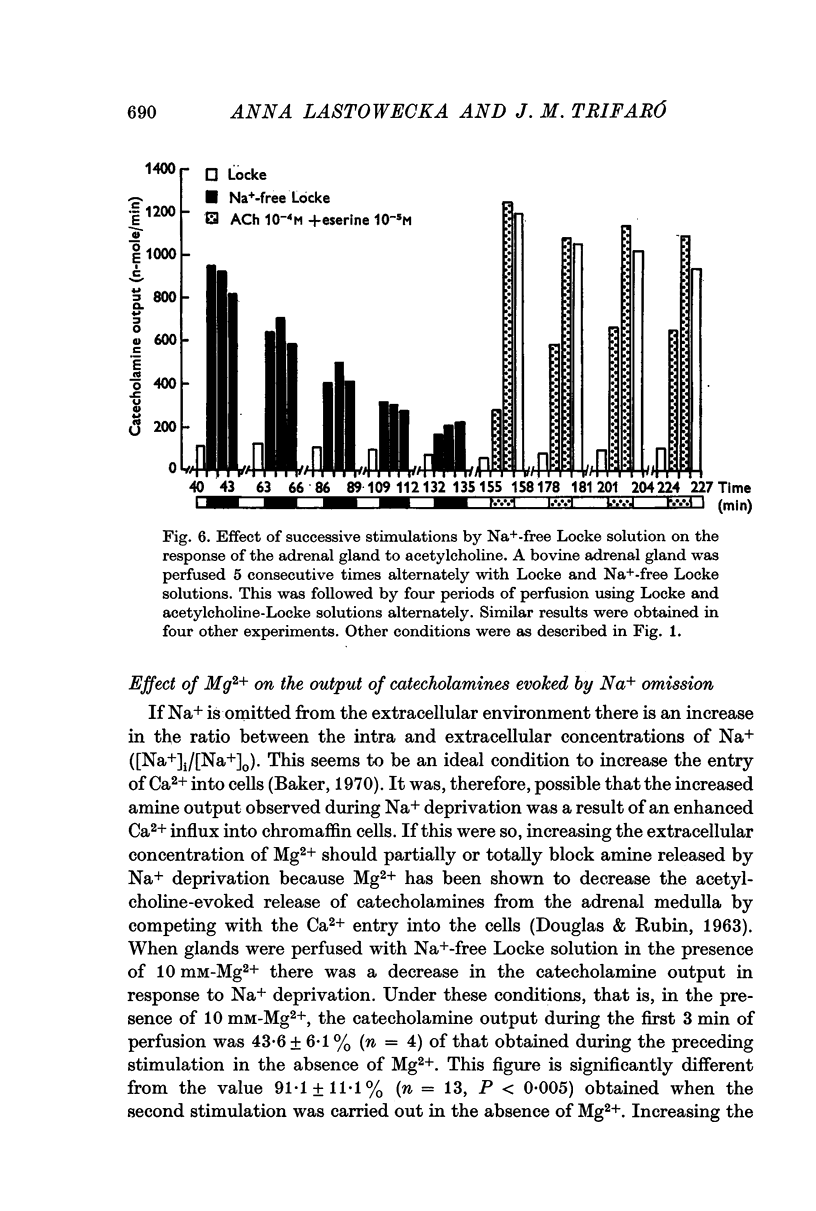
1. Perfusing bovine adrenal glands with Na+-free Locke solution for 15-40 min did not modify the increase in the release of catecholamines from glands stimulated by acetylcholine. However, after 80-100 min of perfusion with Na+-free solution, the response to acetylcholine stimulation was decreased or abolished.
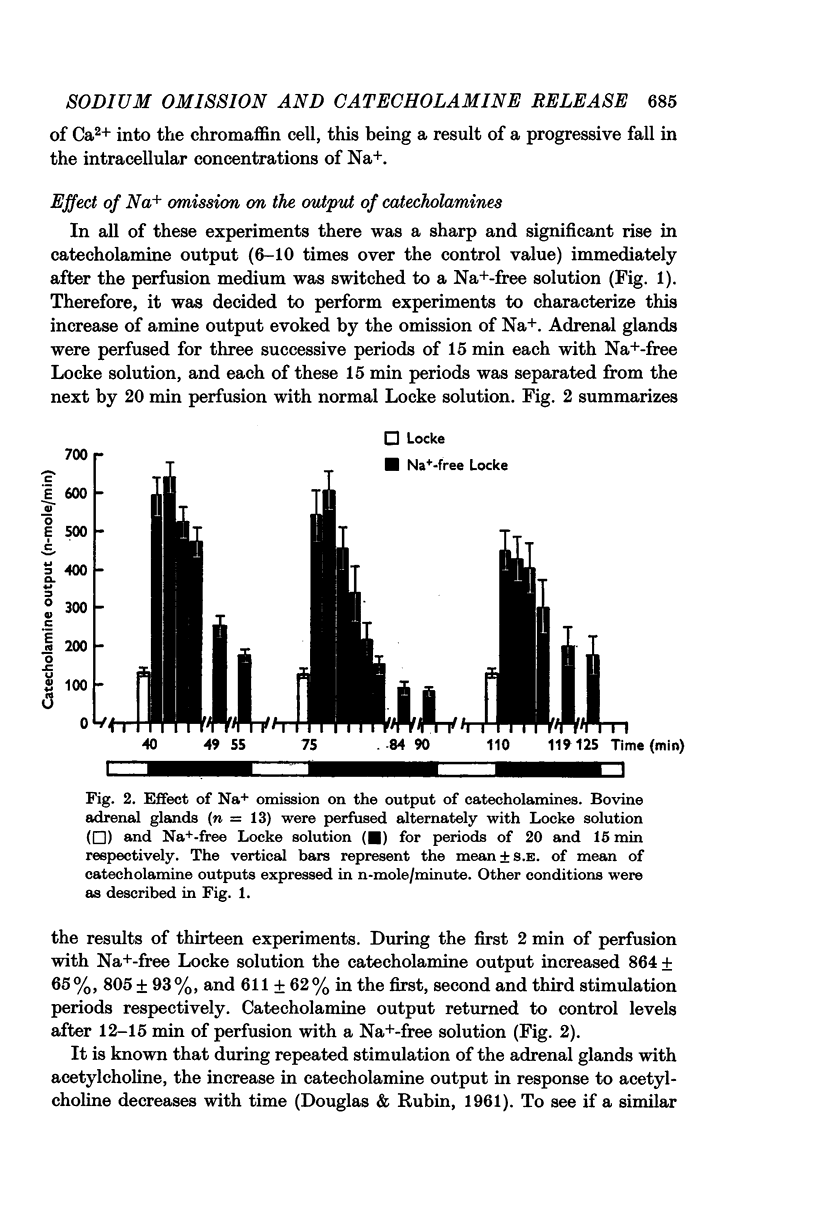
2. Immediately after switching the perfusion medium to Na+-free solution, there was a sharp increase (6-10 times over control values) in catecholamine output.
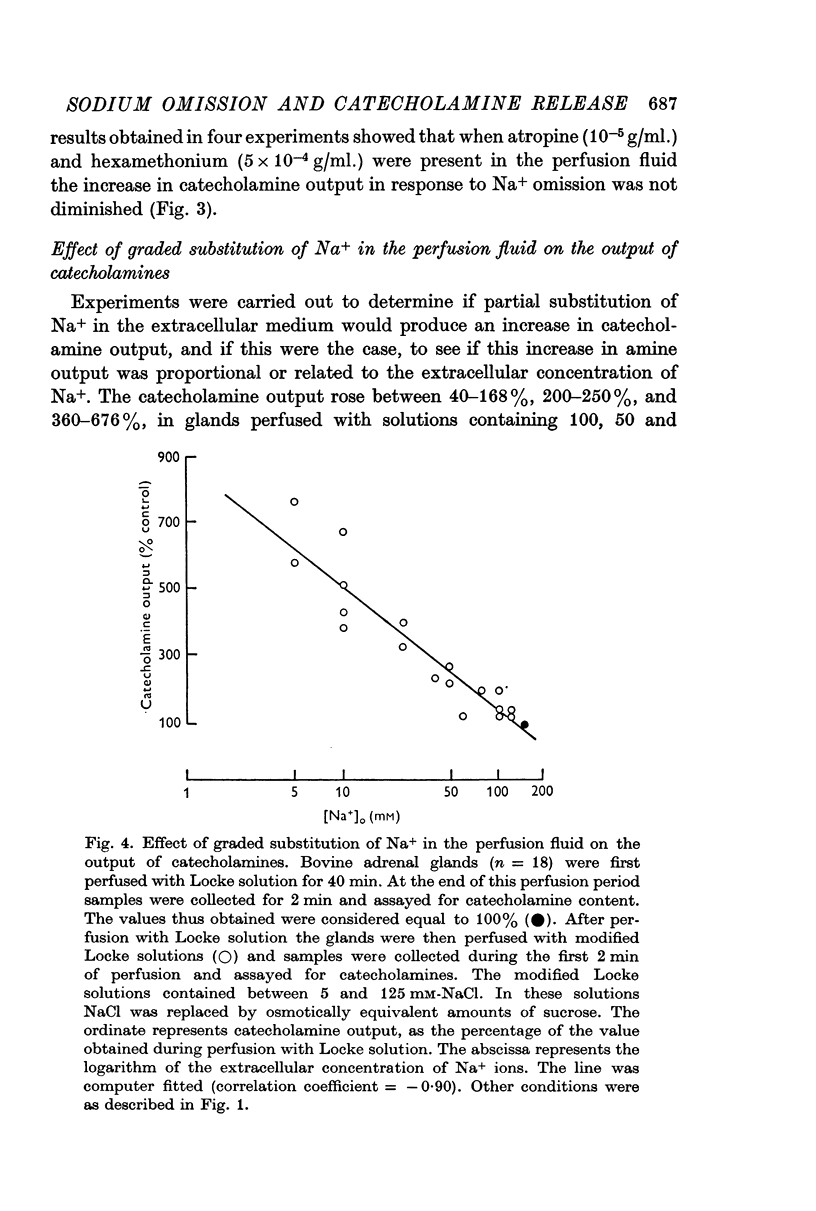
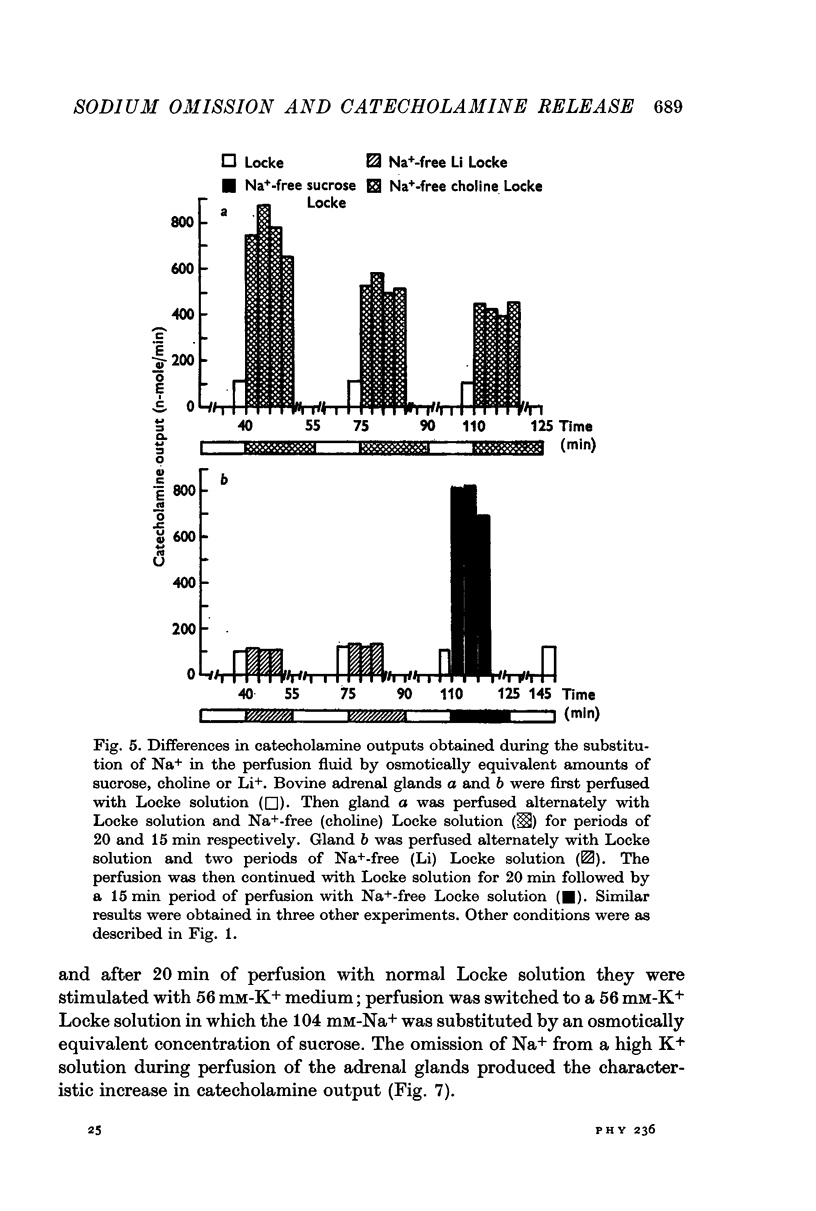
3. Graded substitution of Na+ in the perfusion fluid enhanced the output of catecholamines. This increase in the output of amines was linearly related to the logarithm of the extracellular Na+ concentration.
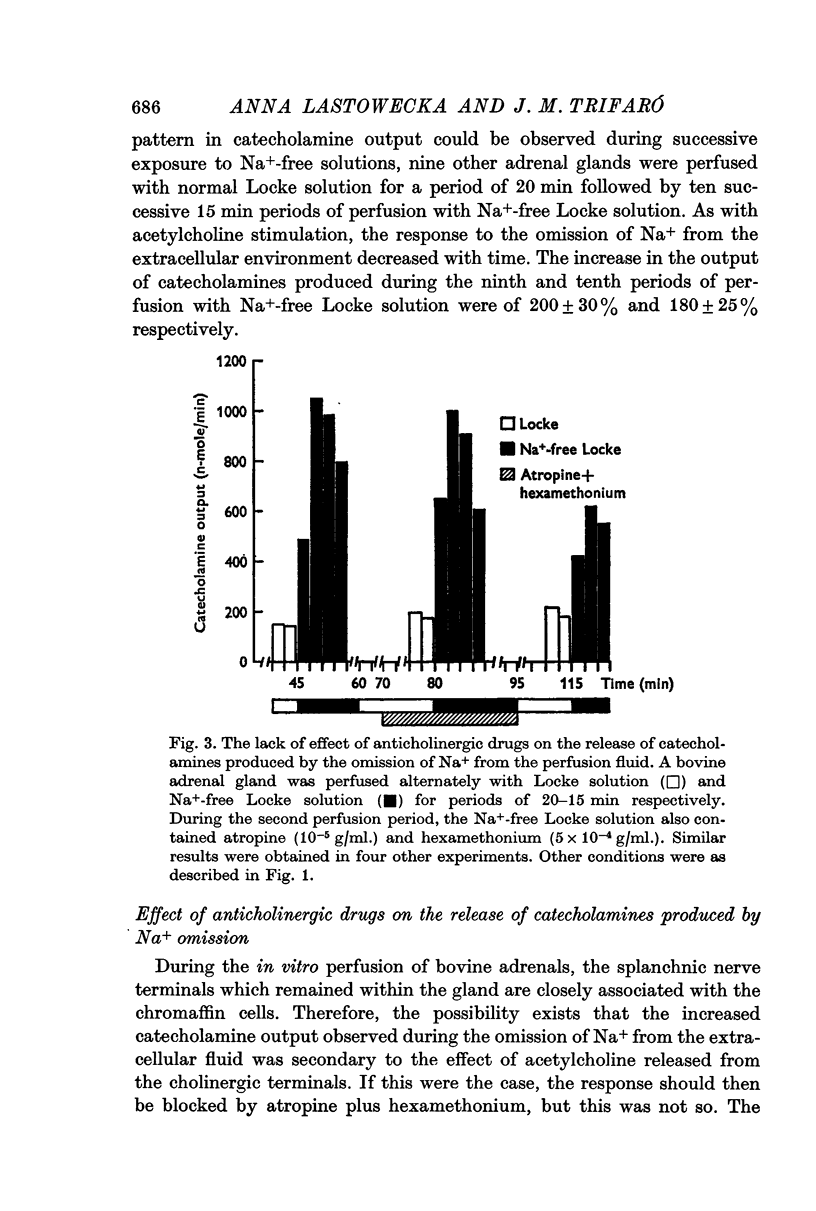
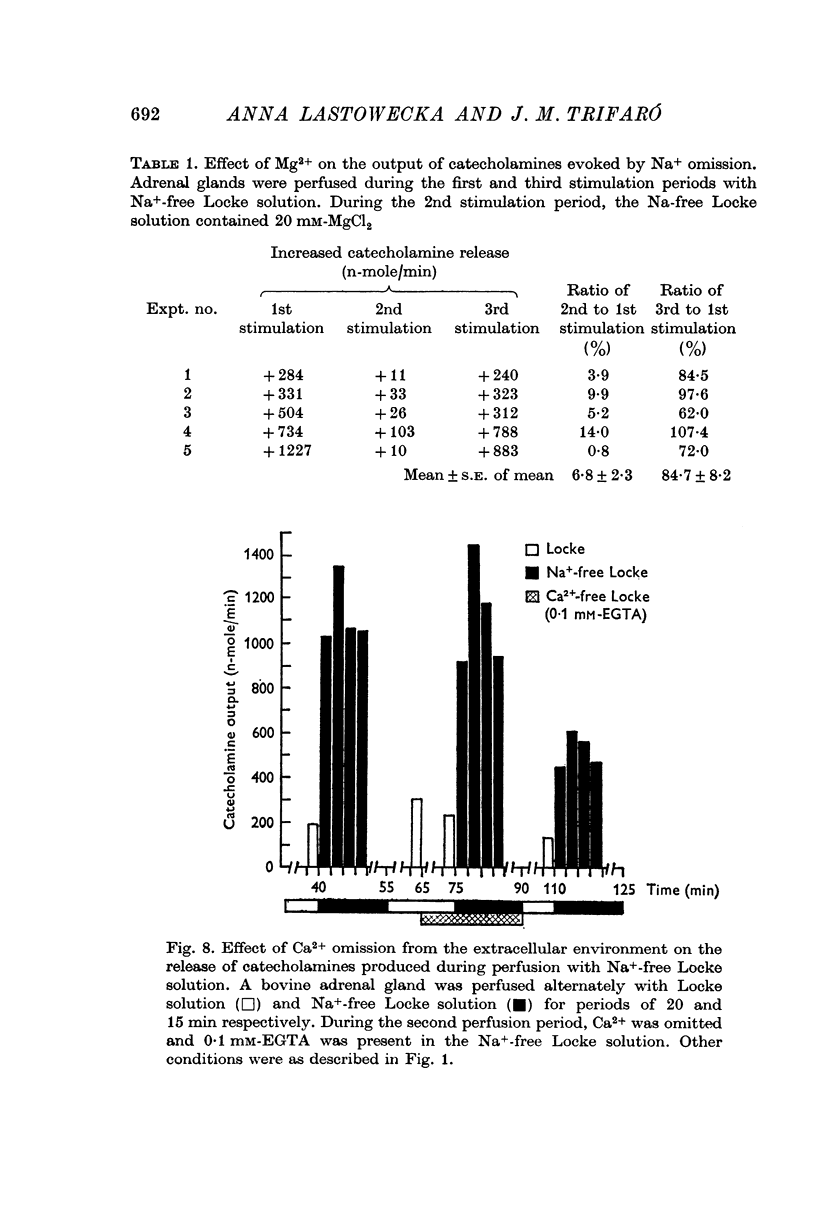
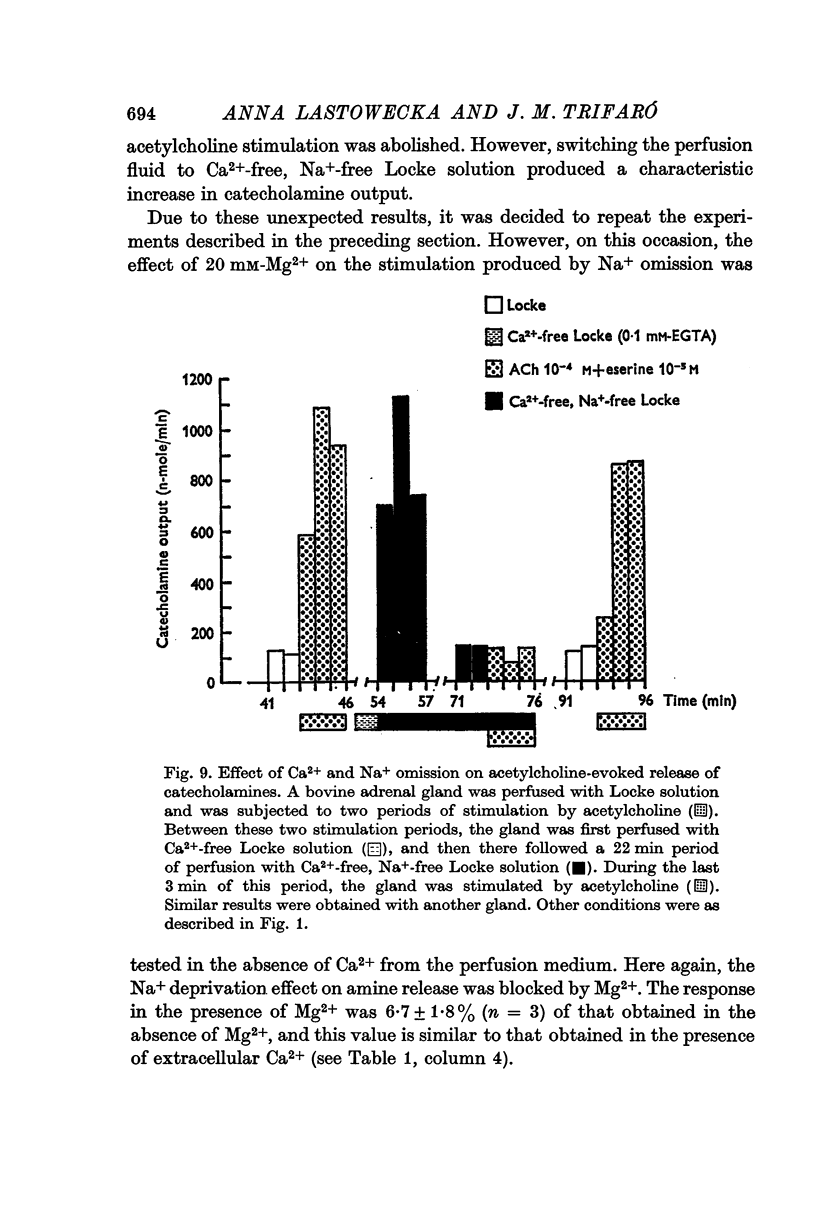
4. The release of catecholamines in the absence of Na+ was not reduced by the presence of atropine and hexamethonium nor by the omission of Ca2+ in the presence of EDTA or EGTA.
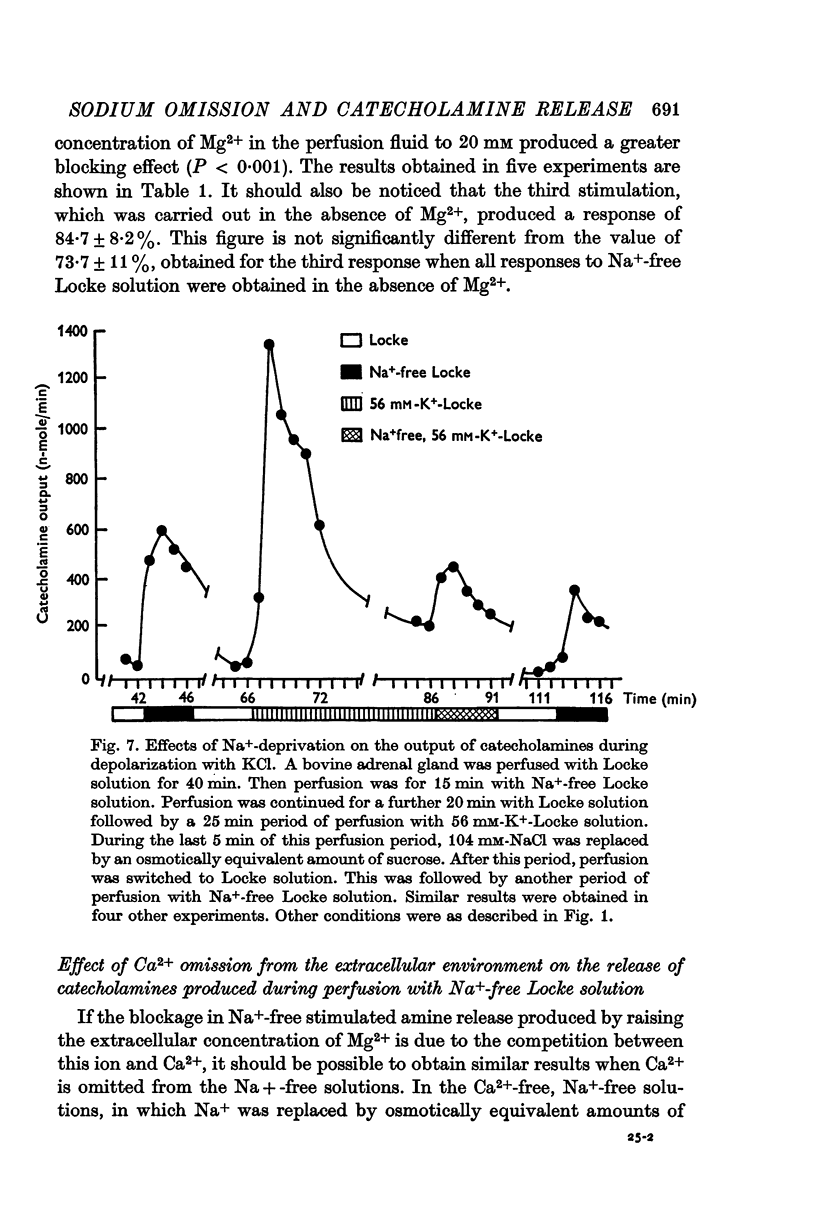
5. Excess of Mg2+ in the perfusion fluid reduced (10 mM-Mg2+) or blocked (20 mM-Mg2+) the increase in the output of catecholamines induced by Na+ deprivation in the presence or absence of extracellular Ca2+.
6. Na+ deprivation induced release of catecholamines during perfusion of the glands with depolarizing concentrations (56 mM) of K+.
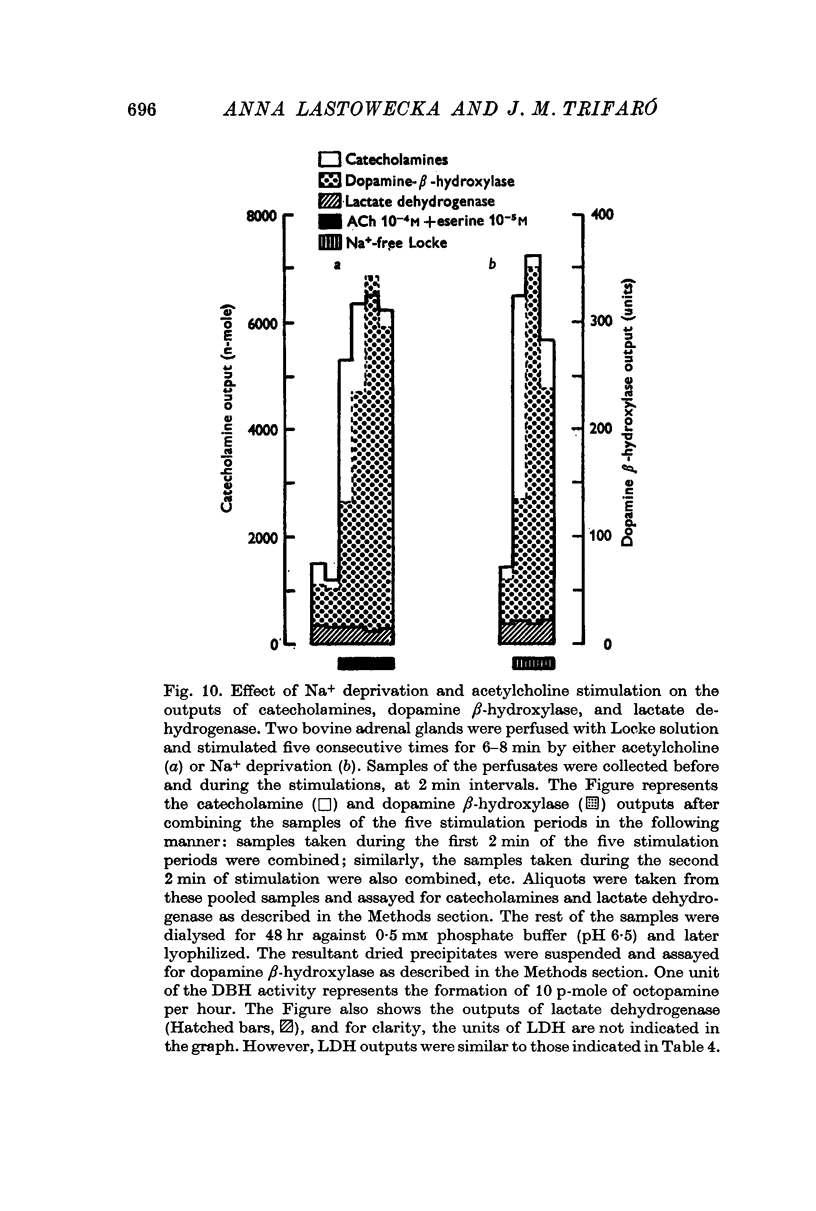
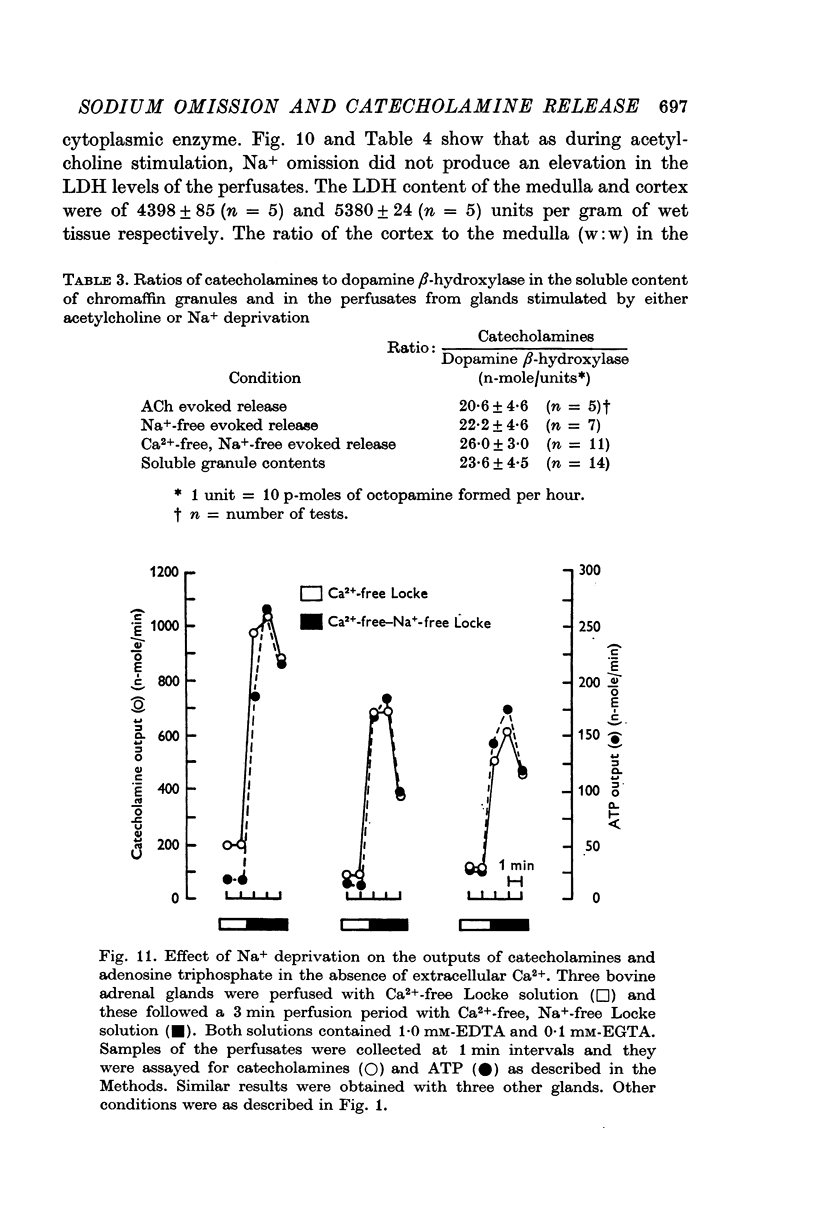
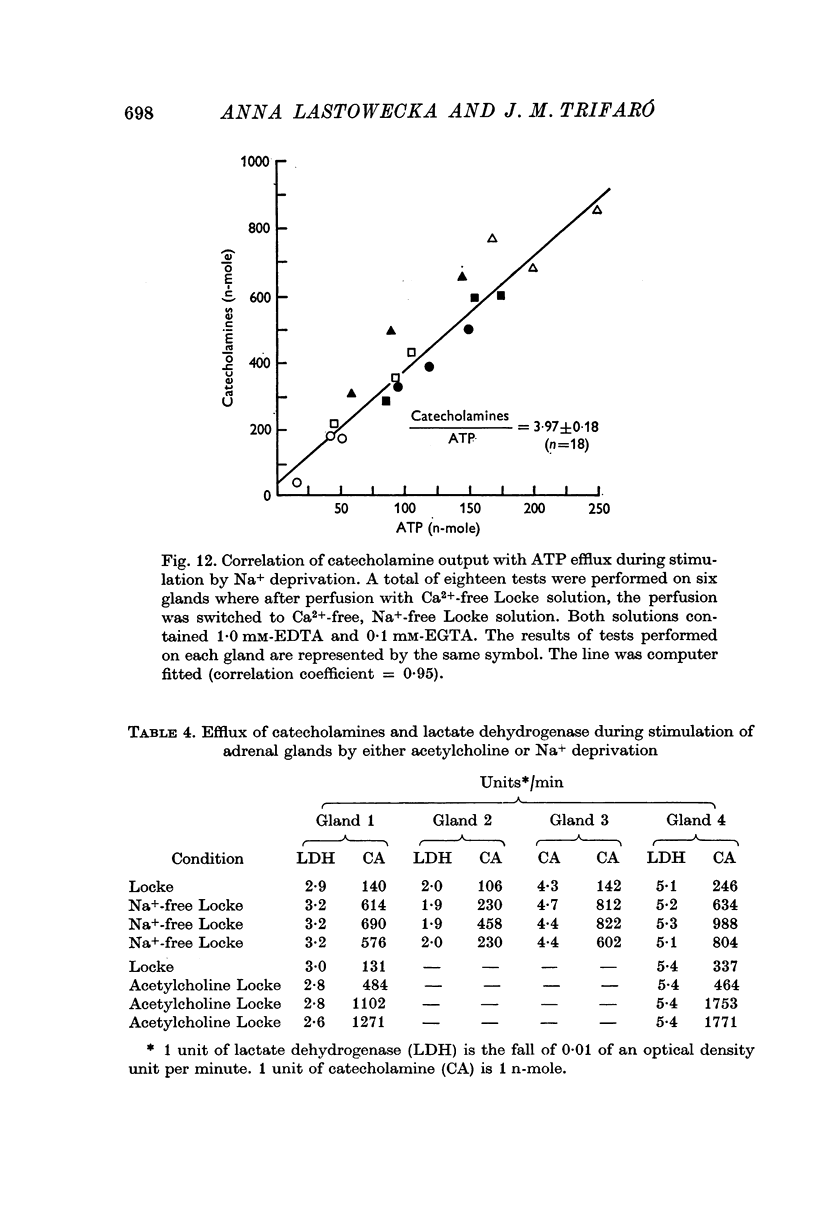
7. In the presence or the absence of extracellular Ca2+, the increase in the output of catecholamines induced by Na+ deprivation was accompanied by an increase in the output of dopamine β-hydroxylase, but not of lactate dehydrogenase. In addition, during perfusion with Ca2+ free solution, Na+ deprivation induced a parallel increase in both catecholamine and adenosine triphosphate outputs.
8. The ratios of catecholamines to dopamine β-hydroxylase and catecholamines to adenosine triphosphate determined in the perfusates collected from glands during perfusion with Na+-free medium were similar to those measured in the soluble contents of isolated chromaffin granules. These results provided biochemical evidence in favour of exocytosis as the mechanism of secretion during Na+ deprivation.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Banks P., Biggins R., Bishop R., Christian B., Currie N. Sodium ions and the secretion of catecholamines. J Physiol. 1969 Feb;200(3):797–805. doi: 10.1113/jphysiol.1969.sp008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P. The effect of ouabain on the secretion of catecholamines and on the intracellular concentration of potassium. J Physiol. 1967 Dec;193(3):631–637. doi: 10.1113/jphysiol.1967.sp008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Evidence that the secreting adrenal chromaffin cell releases catecholamines directly from ATP-rich granules. J Physiol. 1966 Mar;183(1):236–248. doi: 10.1113/jphysiol.1966.sp007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. On the relation between ATP splitting and secretion in the adrenal chromaffin cell: extrusion of ATP (unhydrolysed) during release of catecholamines. J Physiol. 1966 Mar;183(1):249–256. doi: 10.1113/jphysiol.1966.sp007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S., Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity. J Biol Chem. 1965 Dec;240(12):4763–4773. [PubMed] [Google Scholar]
- Gimblet E. G., Marney A. F., Bonsnes R. W. Determination of calcium and magnesium in serum, urine, diet, and stool by atomic absorption spectrophotometry. Clin Chem. 1967 Mar;13(3):204–214. [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S., Zar M. A. The mechanism of acetylcholine release from parasympathetic nerves. J Physiol. 1971 Jul;215(3):819–848. doi: 10.1113/jphysiol.1971.sp009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisner A. M., Hava M. The role of adenosine triphosphate and adenosine triphosphatase in the release of catecholamines from the adrenal medulla. IV. Adenosine triphosphate-- activated uptake of calcium by microsomes and mitochondria. Mol Pharmacol. 1970 Jul;6(4):407–415. [PubMed] [Google Scholar]
- Poisner A. M., Trifaró J. M., Douglas W. W. The fate of the chromaffin granule during catecholamine release from the adrenal medulla. II. Loss of protein and retention of lipid in subcellular fractions. Biochem Pharmacol. 1967 Nov;16(11):2101–2108. doi: 10.1016/0006-2952(67)90007-x. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Trifaró J. M. The role of ATP and ATPase in the release of catecholamines from the adrenal medulla. I. ATP-evoked release of catecholamines, ATP, and protein from isolated chromaffin granules. Mol Pharmacol. 1967 Nov;3(6):561–571. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Schneider F. H., Smith A. D., Winkler H. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother. 1967 Sep;31(1):94–104. doi: 10.1111/j.1476-5381.1967.tb01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró J. M., Dworkind J. A new and simple method for isolation of adrenal chromaffin granules by means of an isotonic density gradient. Anal Biochem. 1970 Apr;34(2):403–412. doi: 10.1016/0003-2697(70)90125-9. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Poisner A. M., Douglas W. W. The fate of the chromaffin granule during catecholamine release from the adrenal medulla. I. Unchanged efflux of phospholipid and cholesterol. Biochem Pharmacol. 1967 Nov;16(11):2095–2100. doi: 10.1016/0006-2952(67)90006-8. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Poisner A. M. The role of ATP and ATPase in the release of catecholamines from the adrenal medulla. II. ATP-evoked fall in optical density of isolated chromaffin granules. Mol Pharmacol. 1967 Nov;3(6):572–580. [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Connett R. J., Kirshner N. Mechanism of secretion from the adrenal medulla. 3. Studies of dopamine beta-hydroxylase as a marker for catecholamine storage vesicle membranes in rabbit adrenal glands. Mol Pharmacol. 1969 Jan;5(1):60–68. [PubMed] [Google Scholar]
- Vizi E. S. Stimulation, by inhibition of (Na + -K + -Mg 2+ )-activated ATP-ase, of acetylcholine release in cortical slices from rat brain. J Physiol. 1972 Oct;226(1):95–117. doi: 10.1113/jphysiol.1972.sp009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Hörtnagl H., Smith A. D. Membranes of the adrenal medulla. Behaviour of insoluble proteins of chromaffin granules on gel electrophoresis. Biochem J. 1970 Jun;118(2):303–310. doi: 10.1042/bj1180303. [DOI] [PMC free article] [PubMed] [Google Scholar]


