Abstract
1. A study has been made of the formation of synapses in the superior cervical ganglion of the guinea-pig, during reinnervation either with axons of the cervical sympathetic trunk, or with somatic axons of the nerve to the sternohyoid muscle.
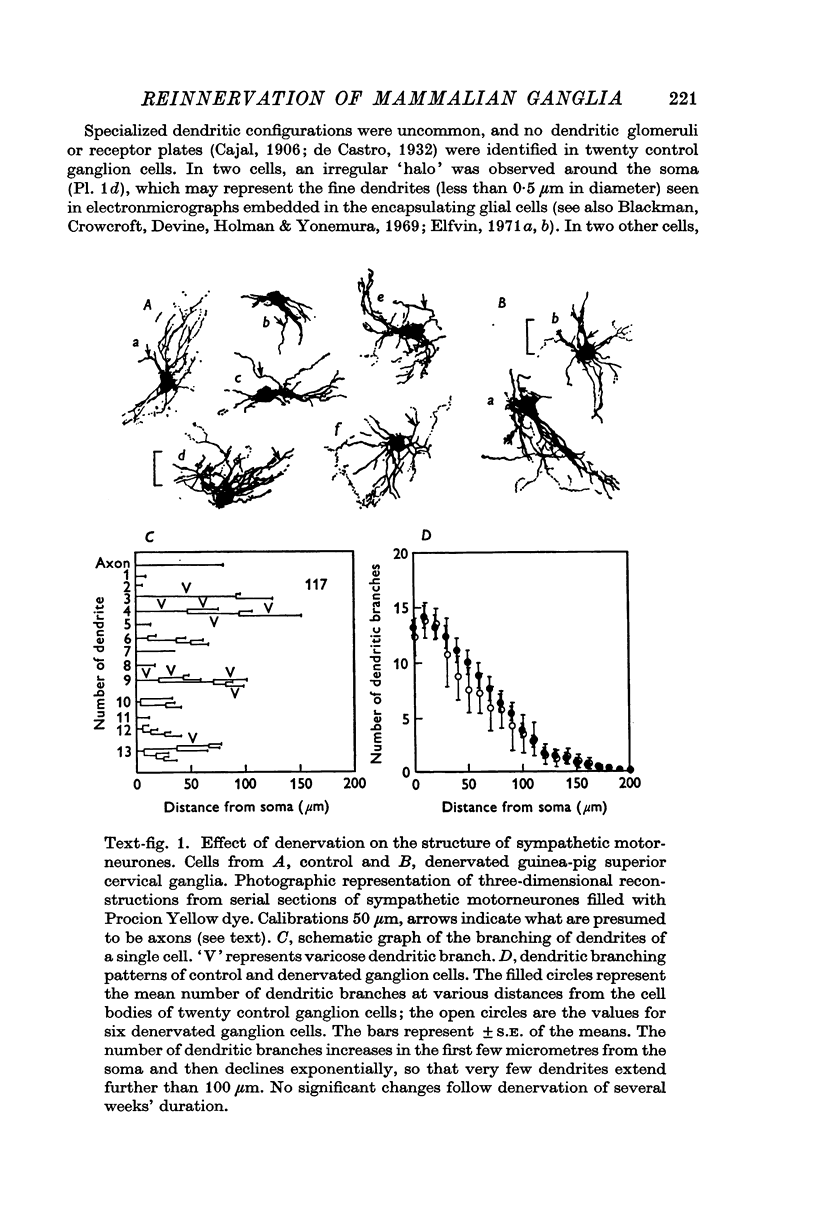
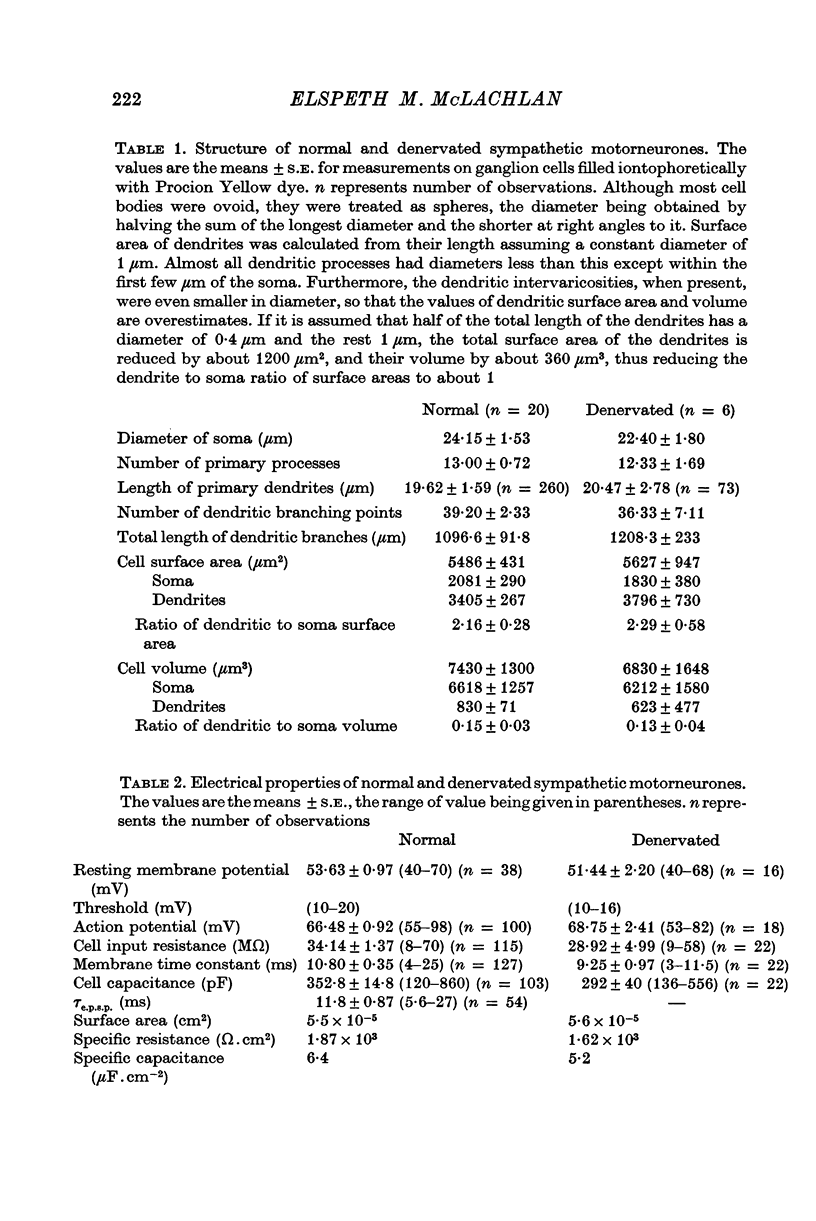
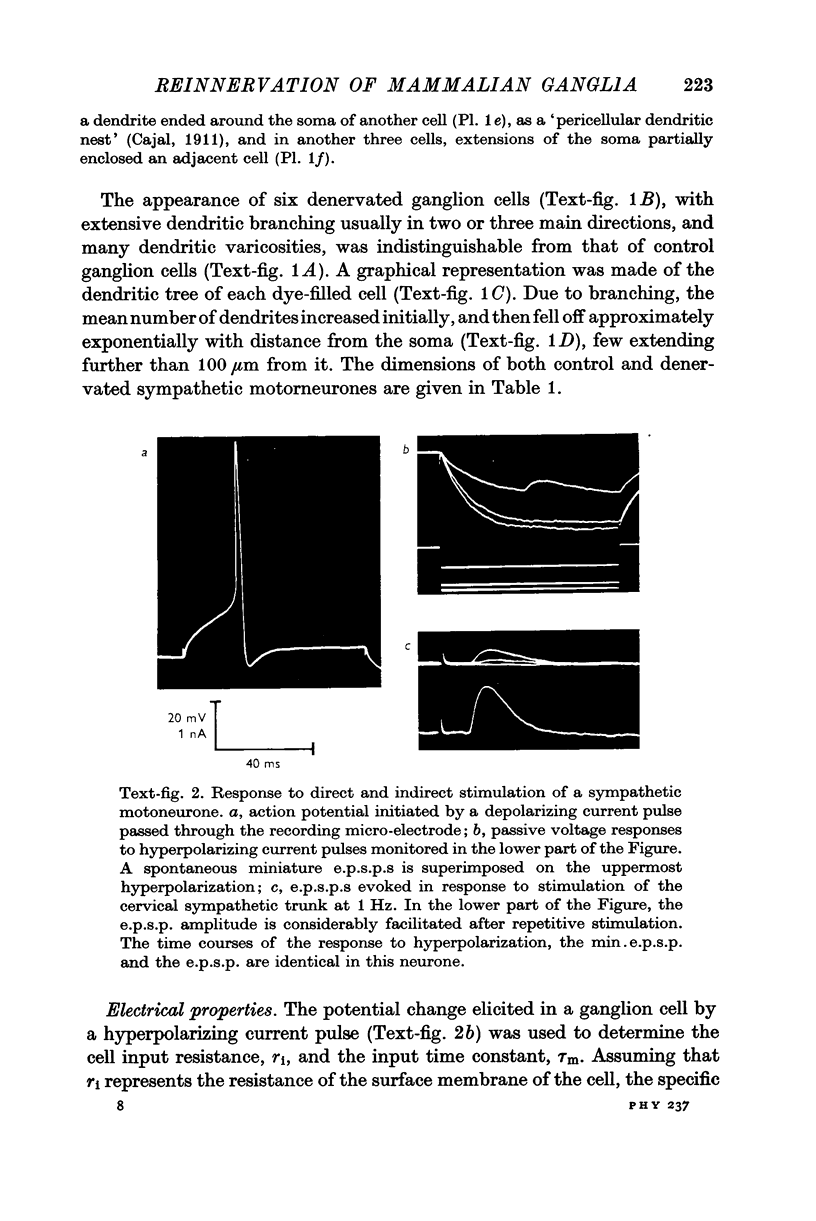
2. No significant changes in either the geometry or electrical parameters of sympathetic motorneurones were detected following denervation for periods of 3-6 weeks, or after reinnervation with either preganglionic or somatic axons.
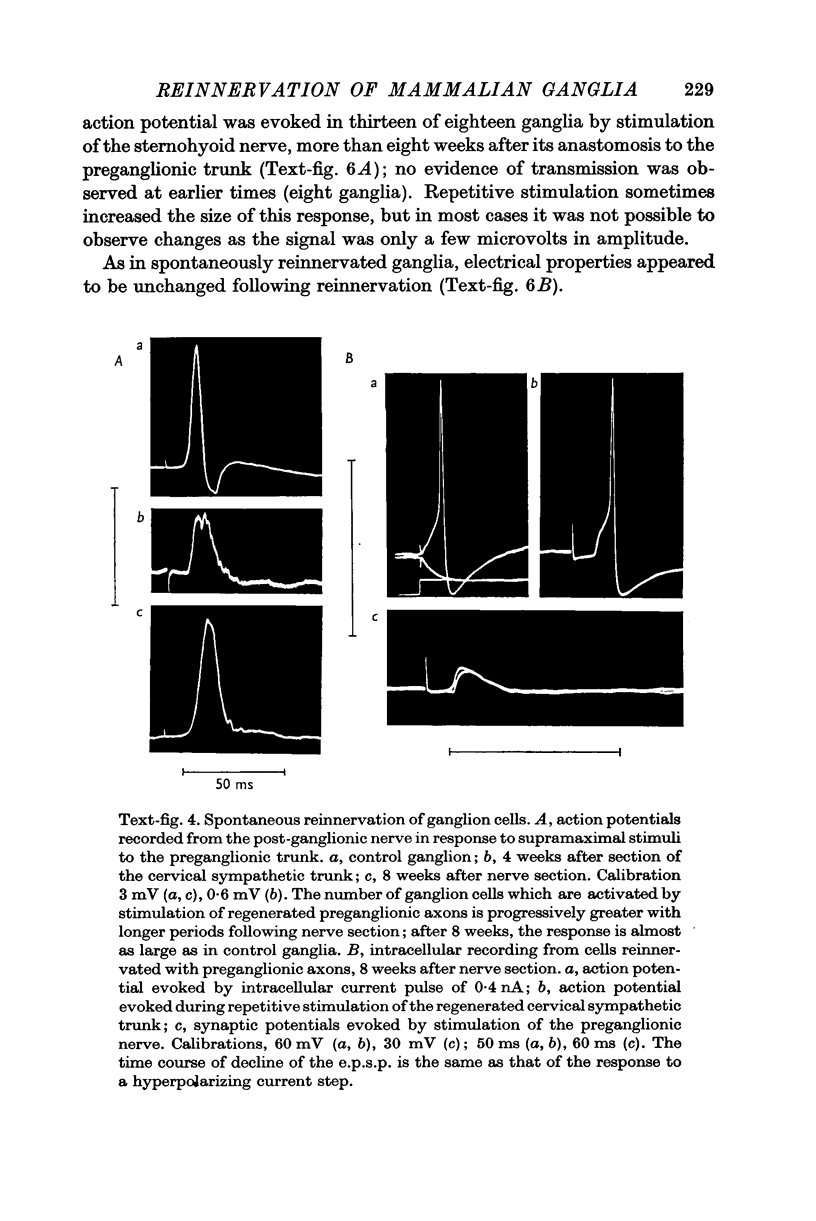
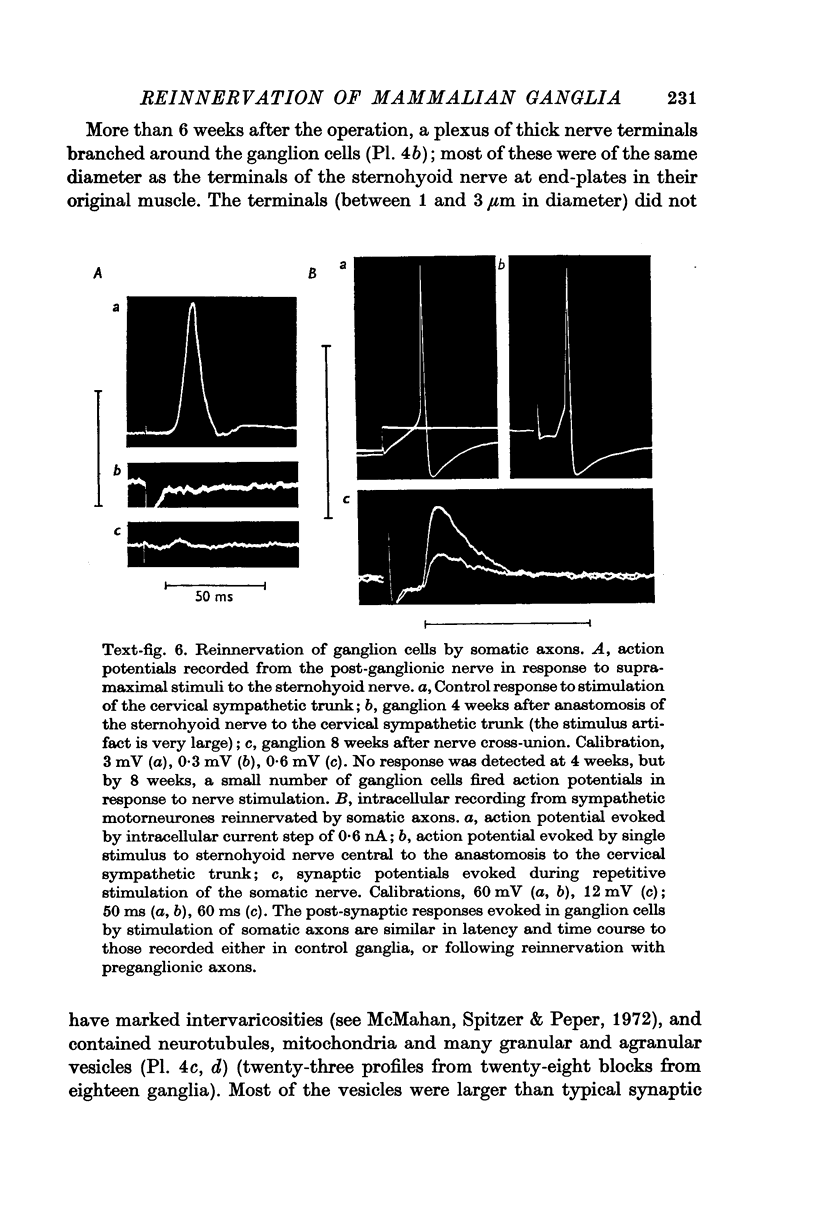
3. The post-ganglionic action potential reappeared about 4 weeks after preganglionic trunk section (eighteen of eighteen ganglia); its amplitude increased progressively and was almost normal by more than 10 weeks after nerve section. A very small response was detected from thirteen of eighteen ganglia after periods longer than 8 weeks after cross-reinnervation with somatic axons.
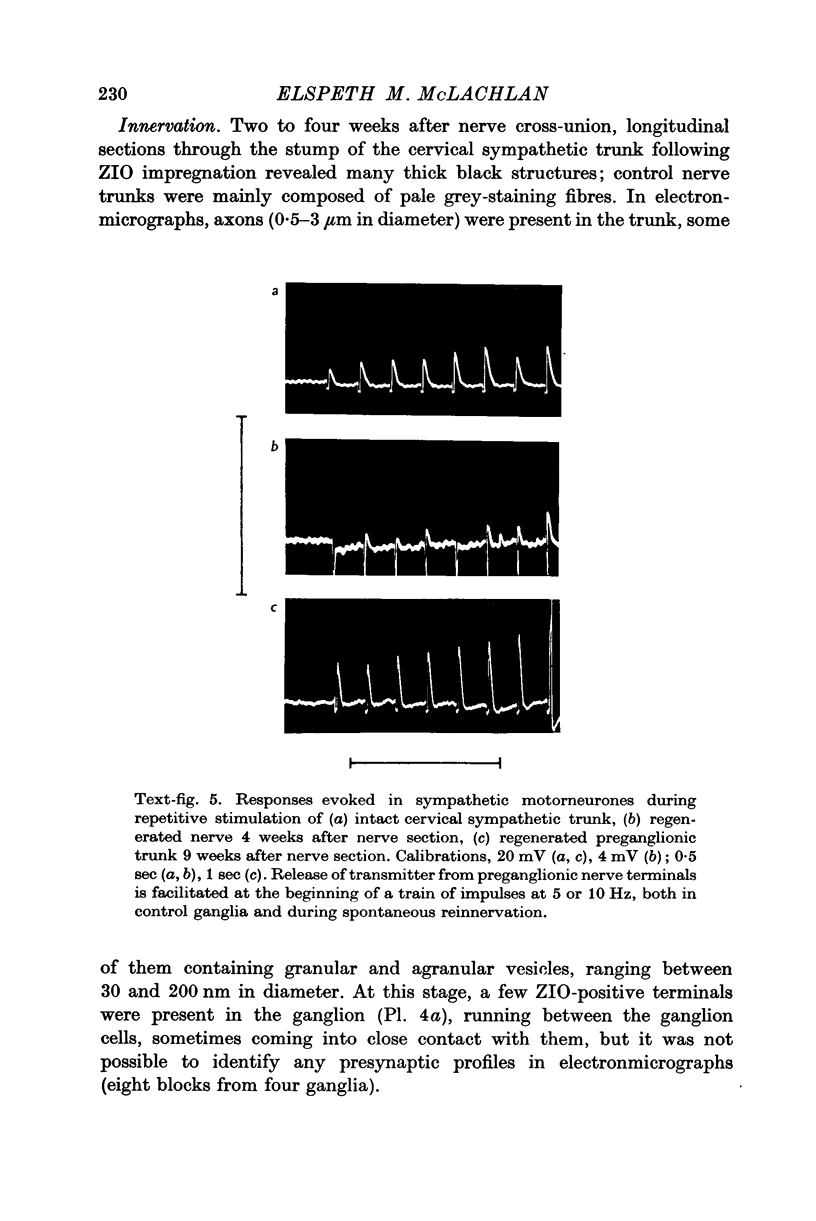
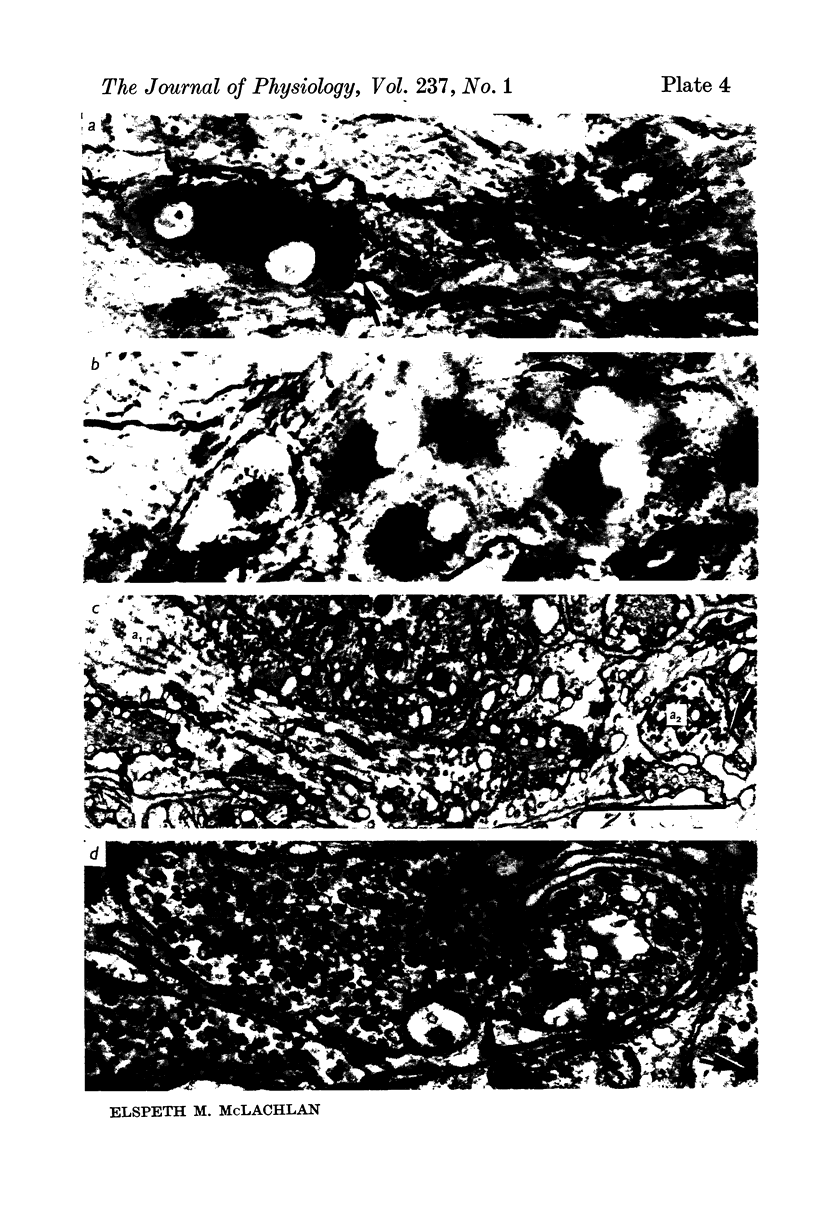
4. Regenerated preganglionic or somatic nerve terminals were demonstrated around the ganglion cells using ZIO impregnation and electron-microscopy; the structure of these terminals was unchanged following regeneration into the ganglia, although many more synapses were formed by preganglionic terminals than by somatic terminals.
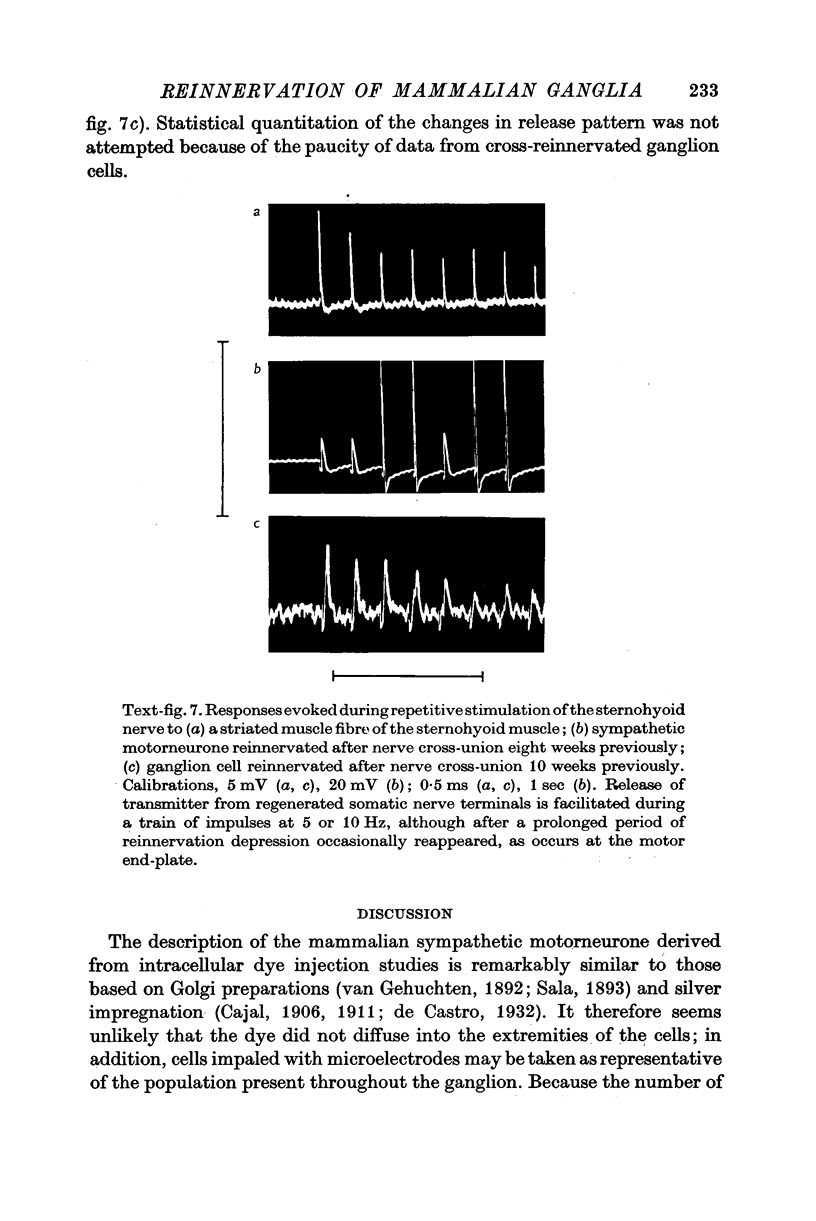
5. The facilitation of evoked synaptic potentials which occurs during repetitive stimulation of preganglionic axons was retained following their regeneration, whereas most synapses formed on ganglion cells by regenerating somatic axons showed facilitation of transmitter release during trains of stimuli, rather than the normal depression.
6. These observations suggest that the structure and electrical properties of adult mammalian autonomic motorneurones are not under neural control, but that these neurones do show some selectivity in the type of nerve which they will permit to form synapses on them.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Sandri C. An electron-microscopic study of zinc iodide-osmium impregnation of neurons. I. Staining of synaptic vesicles at cholinergic junctions. Brain Res. 1968 Feb;7(2):286–295. doi: 10.1016/0006-8993(68)90104-2. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in mammalian striated muscle reinnervated with autonomic preganglionic nerves. J Physiol. 1973 Sep;233(3):501–517. doi: 10.1113/jphysiol.1973.sp010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J. J., Bernstein M. E. Axonal regeneration and formation of synapses proximal to the site of lesion following hemisection of the rat spinal cord. Exp Neurol. 1971 Feb;30(2):336–351. doi: 10.1016/s0014-4886(71)80012-2. [DOI] [PubMed] [Google Scholar]
- Bernstein M. E., Bernstein J. J. Regeneration of axons and synaptic complex formation rostral to the site of hemisection in the spinal cord of the monkey. Int J Neurosci. 1973 Jan;5(1):15–26. doi: 10.3109/00207457309149445. [DOI] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Peterson E. R. The onset of synapse formation in spinal cord cultures as studied by electron microscopy. Brain Res. 1967 Dec;6(4):728–749. doi: 10.1016/0006-8993(67)90129-1. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Clementi F., Mantegazza P. Synaptic transmission in the superior cervical ganglion of the cat after reinnervation by vagus fibres. J Physiol. 1971 Jul;216(1):87–98. doi: 10.1113/jphysiol.1971.sp009510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESMEDT J. E. The physio-pathology of neuromuscular transmission and the trophic influence of motor innervation. Am J Phys Med. 1959 Dec;38:248–261. [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Elfvin L. G. Ultrastructural studies on the synaptology of the inferior mesenteric ganglion of the cat. I. Observations on the cell surface of the postganglionic perikarya. J Ultrastruct Res. 1971 Nov;37(3):411–425. doi: 10.1016/s0022-5320(71)80135-1. [DOI] [PubMed] [Google Scholar]
- Engel E., Barcilon V., Eisenberg R. S. The interpretation of current-voltage relations recorded from a spherical cell with a single microelectrode. Biophys J. 1972 Apr;12(4):384–403. doi: 10.1016/S0006-3495(72)86091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. H. Endings produced by somatic nerve fibres growing into the adrenal gland. J Anat. 1947 Jul;81(Pt 3):225–232.2. [PMC free article] [PubMed] [Google Scholar]
- Govind C. K., Atwood H. L., Lang F. Synaptic differentiation in a regenerating crab-limb muscle. Proc Natl Acad Sci U S A. 1973 Mar;70(3):822–826. doi: 10.1073/pnas.70.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., NELSON P. G. STRUCTURAL AND FUNCTIONAL CHANGES IN THE FROG SYMPATHETIC GANGLION FOLLOWING CUTTING OF THE PRESYNAPTIC NERVE FIBRES. J Physiol. 1965 Mar;177:1–20. doi: 10.1113/jphysiol.1965.sp007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hámori J., Láng E., Simon L. Experimental degeneration of the preganglionic fibers in the superior cervical ganglion of the cat. An electron microscope study. Z Zellforsch Mikrosk Anat. 1968;90(1):37–52. doi: 10.1007/BF00496701. [DOI] [PubMed] [Google Scholar]
- JONES W. H., THOMAS D. B. Changes in the dendritic organization of neurons in the cerebral cortex following deafferentation. J Anat. 1962 Jul;96:375–381. [PMC free article] [PubMed] [Google Scholar]
- James D. W., Tresman R. L. An electron-microscopic study of the de novo formation of neuromuscular junctions in tissue culture. Z Zellforsch Mikrosk Anat. 1969;100(1):126–140. doi: 10.1007/BF00343826. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The fine structure of motor endplate morphogenesis. J Cell Biol. 1969 Jul;42(1):154–169. doi: 10.1083/jcb.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Dennis M. J., Harris A. J. The development of chemosensitivity in extrasynaptic areas of the neuronal surface after denervation of parasympathetic ganglion cells in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):555–563. doi: 10.1098/rspb.1971.0047. [DOI] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. Pharmacological properties, cholinesterase activity and anatomy of nerve-muscle junctions in vagus-innervated frog sartorius. J Physiol. 1972 Jan;220(1):243–256. doi: 10.1113/jphysiol.1972.sp009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. On the union of the fifth cervical nerve with the superior cervical ganglion. J Physiol. 1904 Feb 25;30(5-6):439–442. doi: 10.1113/jphysiol.1904.sp001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The union of different kinds of nerve fibres. J Physiol. 1904 Aug 22;31(5):365–391. doi: 10.1113/jphysiol.1904.sp001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the Regeneration of Pre-Ganglionic and of Post-Ganglionic Visceral Nerve Fibres. J Physiol. 1897 Nov 20;22(3):215–230. doi: 10.1113/jphysiol.1897.sp000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the Union of Cranial Autonomic (Visceral) Fibres with the Nerve Cells of the Superior Cervical Ganglion. J Physiol. 1898 Jul 26;23(3):240–270. doi: 10.1113/jphysiol.1898.sp000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser J. D., Ward A. A., Jr Some effects of deafferentation on neurons of the cat spinal cord. Arch Neurol. 1967 Dec;17(6):629–636. doi: 10.1001/archneur.1967.00470300071012. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. The regeneration of neuromuscular junctions during spontaneous re-innervation of the rat diaphragm. Z Zellforsch Mikrosk Anat. 1971;121(4):593–603. doi: 10.1007/BF00560162. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS M. R., POWELL T. P. Some observations on transneuronal cell degeneration in the olfactory bulb of the rabbit. J Anat. 1962 Jan;96:89–102. [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. A study of the reinnervation of fast and slow mammalian muscles. J Gen Physiol. 1973 Jan;61(1):1–23. doi: 10.1085/jgp.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J. Fine structure of synapses in the dorsal nucleus of the lateral geniculate body of normal and blinded rats. Z Zellforsch Mikrosk Anat. 1967;76(1):116–146. doi: 10.1007/BF00337036. [DOI] [PubMed] [Google Scholar]
- Morest D. K. The growth of dendrites in the mammalian brain. Z Anat Entwicklungsgesch. 1969;128(4):290–317. doi: 10.1007/BF00522529. [DOI] [PubMed] [Google Scholar]
- Pellegrino de Iraldi A., De Robertis E. The neurotubular system of the axon and the origin of granulated and non-granulated vesicles in regenerating nerves. Z Zellforsch Mikrosk Anat. 1968;87(3):330–344. doi: 10.1007/BF00333684. [DOI] [PubMed] [Google Scholar]
- RALL W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960 Oct;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. D. The qnit membrane of cells and mechanisms of myelin formation. Res Publ Assoc Res Nerv Ment Dis. 1962;40:94–158. [PubMed] [Google Scholar]
- SHOLL D. A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953 Oct;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- SJOQVIST F. The correlation between the occurrence and localization of acetylcholinesterase-rich cell bodies in the stellate ganglion and the outflow of cholinergic sweat secretory fibres to the fore paw of the cat. Acta Physiol Scand. 1963 Apr;57:339–351. doi: 10.1111/j.1748-1716.1963.tb02597.x. [DOI] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- Sherman R. G., Atwood H. L. Correlated electrophysiological and ultrastructural studies of a crustacean motor unit. J Gen Physiol. 1972 May;59(5):586–615. doi: 10.1085/jgp.59.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. A., Young J. Z. Regeneration of fibre diameter after cross-unions of visceral and somatic nerves. J Anat. 1945 Apr;79(Pt 2):48–65. [PMC free article] [PubMed] [Google Scholar]
- Sotelo C. Permanence of postsynaptic specializations in the frog sympathetic ganglion cells after denervation. Exp Brain Res. 1968;6(4):294–305. doi: 10.1007/BF00233181. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kravitz E. A. Neuronal geometry: determination with a technique of intracellular dye injection. Science. 1968 Oct 4;162(3849):132–134. doi: 10.1126/science.162.3849.132. [DOI] [PubMed] [Google Scholar]
- Taxi J., Babmindra V. P. Light and electron microscopic studies of normal and heterogeneously regenerated ganglionic synapses of the dog. J Neural Transm. 1972;33(4):257–274. doi: 10.1007/BF01245838. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski H. M., Ghetti B., Horoupian D. S. The fate of synaptic membranes of degenerating optic nerve terminals, and their role in the mechanism of trans-synaptic changes. J Neurocytol. 1972 Oct;1(3):297–310. doi: 10.1007/BF01099940. [DOI] [PubMed] [Google Scholar]