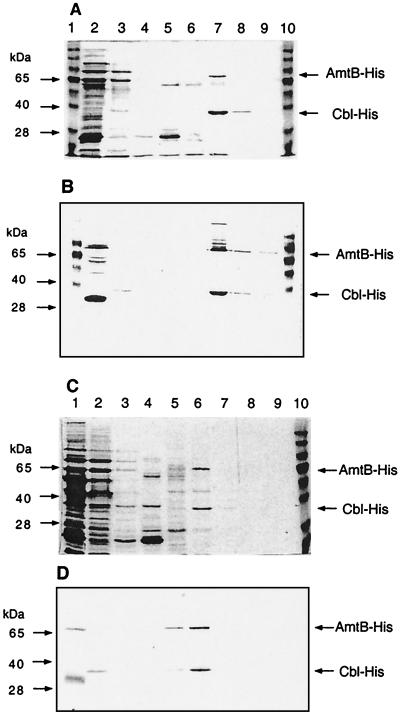
FIG. 2.
Metal affinity purification of A. aeolicus AmtB-His6 and mutant form W32 → stop (see the text). Samples taken during purification (see the text) were subjected to SDS-10% polyacrylamide gel electrophoresis. (A and C) Coomassie blue-stained gels; (B and D) Western blots with anti-His antibody (see the legend to Fig. 1). (A and B) Lane 1, molecular weight standards; lane 2, crude extract after heat treatment; lane 3, flowthrough of Ni-NTA affinity column; lane 4, first wash (in breakage buffer modified to contain 0.1 mM phenylmethylsulfonyl fluoride and 0.1% octylglucoside); lane 5, second wash (in breakage buffer adjusted to pH 6, modified as described above, and containing 10% glycerol); lane 6, eluate with 50 mM imidazole added to the second wash buffer; lane 7, eluate with 200 mM imidazole; lane 8, eluate with 500 mM imidazole; lane 9, eluate with 1,000 mM imidazole; lane 10, molecular weight standards. (C and D) Lane 1, crude extract after heat treatment; lane 2, flowthrough of Ni-NTA affinity column; lane 3, first wash (pH 8.0); lane 4, second wash (pH 6.0); lanes 5 to 8, eluates with 50, 200, 500, and 1,000 mM imidazole, respectively; lane 9, wash with 0.2 M acetic acid; lane 10, molecular weight standards.