Figure 2.
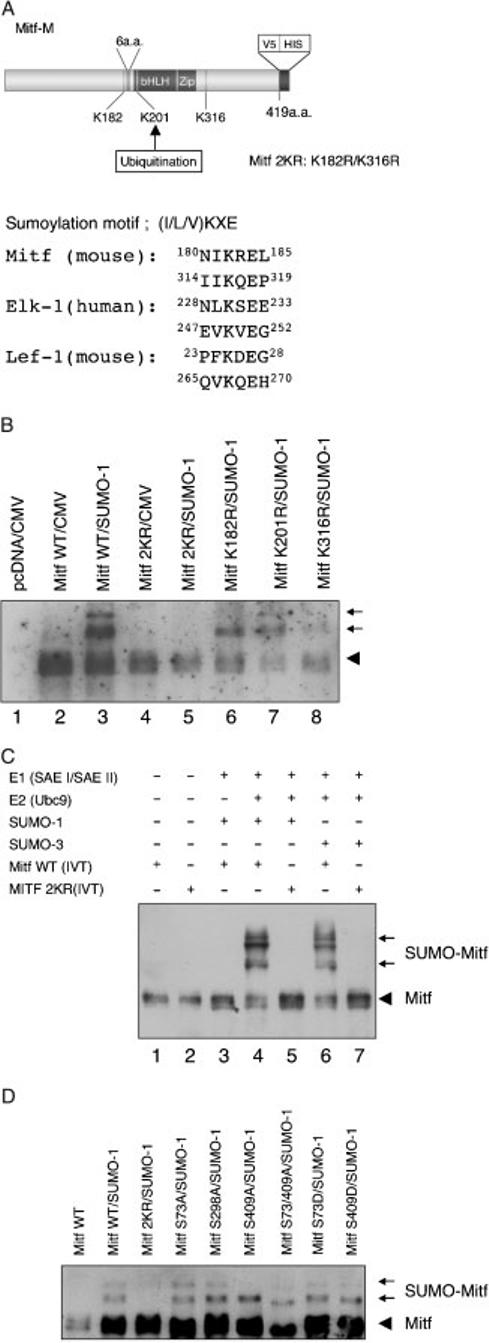
Lysine 182 and lysine 316 are the sites of SUMO-conjugation in MITF in vitro and in vivo. (A) Schematic representation of MITF. Two possible sumoylation sites are present at position 182 and 316. The putative acceptor sites and the alignment of sumoylation motifs in MITF and reference proteins are shown on the right. (B) Protein extracts from cells co-transfected with constructs encoding wild type MITF or MITF with the indicated mutations [K182R, K316R, both K182/316R (2KR) or K201R], and flag-tagged SUMO-1 were immunoprecipitated with anti-V5 antibody. The precipitates were analyzed by immunoblotting with horseradish peroxidase-coupled anti-V5 antibody. The V5-His tagged Mitf is indicated by an arrowhead and its corresponding conjugated forms by arrows. (C) In vitro sumoylation of MITF. In vitro translated wild type and mutant MITF were subjected to in vitro sumoylation using purified recombinant E1 (SAE I/SAE II) enzyme, E2 (Ubc9) enzyme, and recombinant SUMO-1 or SUMO-3 in the presence of ATP. The reaction mixes were subjected to SDS-PAGE and immunoblotted with anti-V5 antibody. Note that in vitro reactions can result in additional MITF forms not detected in transfected cells which may reflect conformational isomers. (D) MITF with mutations in phosphorylation sites are normally sumoylated. The indicated plasmids encoding wild type and mutant MITF were transfected into HEK293 cells along with the SUMO-1 expression plasmid, and cellular extracts subjected to electrophoresis and immunoblotting. Note that only 2KR mutant MITF shows no SUMO-modified forms. Also note that the S73A/409A double mutant, modified or not, shows a faster migration than the other mutants, consistent with previous results (Wu et al., 2000) and independent unpublished observations (Arnheiter et al., in press). Longer exposures (not shown) clearly revealed both mono- and doubly sumoylated forms in wild type MITF and all mutants except 2KR-MITF.
