Abstract
Summary: Functional MR imaging for language lateralization was performed in a 6-year-old child before neurosurgical intervention. A passive story-listening task was used; this revealed a bilateral language network. The task was repeated during the same session when the child had fallen asleep and surprisingly yielded strong activation in similar language areas. Our findings suggest that language processing does occur during natural sleep, even in young children. This potentially allows for an assessment of language functions, even in sleeping children.
Although functional MR imaging has been used with success in young children, it has been limited by their ability to cooperate in the awake state. Earlier studies have demonstrated limited success in studying sensory activation in the sedated child (1, 2) but not during natural sleep. In fact, surprisingly little is known about the brain’s physiology during sleep and, indeed, about the very purpose of sleep itself. The extent to which brain processes during sleep differ from those during the awake state remains unclear. The recent application of functional imaging methods has renewed research interest in this field (3).
We report on a young boy who was referred for functional MR imaging as part of presurgical planning to assess language lateralization. The same language task was conducted while the patient was awake and while he was asleep, with surprising results. The results of this case are compared with data from four healthy 6-year-old boys performing the same task.
Case Report
A 6-year-old right-handed boy was seen by a neurologist for complex partial seizures. MR imaging was performed and demonstrated an enhancing left mesial temporal lobe lesion (Fig 1), which, after resection, was confirmed to be a ganglioglioma. As part of the presurgical evaluation, the child was scheduled for a functional MR study to evaluate the proximity of language function to the left temporal lobe lesion. Internal review board approval and informed consent were obtained before imaging was performed.
Fig 1.
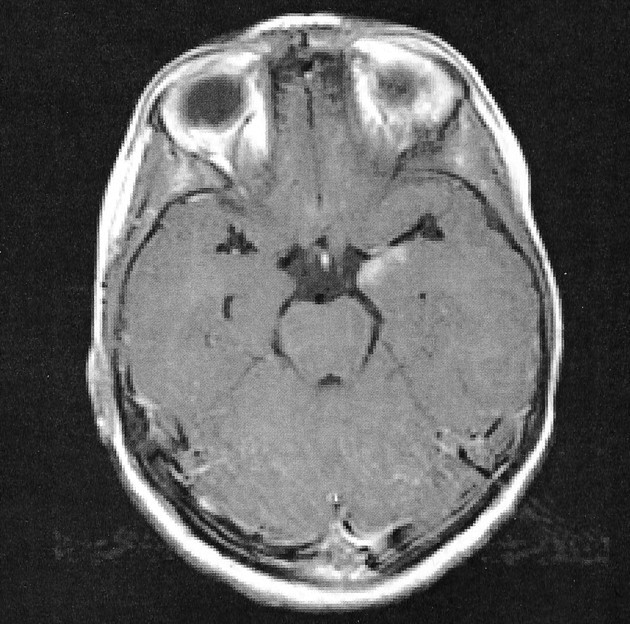
Contrast-enhanced T1-weighted image demonstrates the enchancing lesion in a 6-year-old boy with complex partial seizures in the left temporal lobe.
Imaging was performed with a Biospec 30/60 3-T MR imager equipped with a head gradient insert (Bruker SK330; Bruker Medizintechnik, Ettlingen, Germany). Functional images were acquired by using a gradient-echo T2*-weighted echo-planar sequence (TR/TE, 3000/38; in-plane resolution, 4 × 4 mm; section thickness, 5 mm), yielding 24 sections over 110 time points during the alternating 30-second periods of control and activation. The total imaging time was 5 minutes 30 seconds. A T1-weighted, three-dimensional whole-brain image was also acquired (15/4.3, 1 × 1.5 × 2.25 mm). Postprocessing was performed by using Cincinnati Children’s Hospital Image Processing Software (CCHIPS) and included the removal of Nyquist ghosts and geometric distortions by use of a multiecho reference image (4). Motion correction was accomplished by using C routines. Images were analyzed using a cross-correlation technique.
Because of the age of the child, a passive-listening task (stories vs tones) was performed. Although the child was able to complete the functional task, the motion of his hands and arms during the study impaired the quality of the image. Motion, which reduces the correspondence between images acquired at different points in time, generally degrades the ability to correctly detect functional activation, because it interferes with the ascription of substantial signal intensity changes to a given voxel. To give the child time to settle down, an anatomic image was acquired. When the boy was approached to explain the next task, it was found that he had fallen asleep. It was decided to repeat the study, and he was still asleep when he was removed from the imager bore several minutes later.
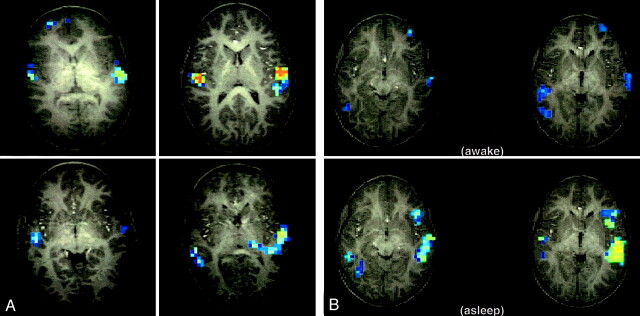
Although the image obtained when the patient was awake revealed limited activation in both the Broca and Wernicke areas in the left cerebral hemisphere (Fig 2B, top row), activation was not only present in similar language areas on the sleep image but also far more brisk (Fig 2B, bottom row).
Fig 2.
Functional MR data obtained in subjects who were performing a story-listening task.
A, Reference data from four healthy 6-year-old boys obtained while they were listening to stories (active condition) or tones (control condition). Note the variability in the activation patterns.
B, Imaging data from our patient who performed the same task while awake (top) and in the sleeping state (bottom). Cross-correlation r=0.375, cluster size=5. Note that, at the same analysis threshold, stronger activation is shown in the sleep study
Discussion
The visualization of eloquent cortical regions near a brain lesion has gained widespread attention, because this information may aid in minimizing postoperative neurologic deficits. Functional MR imaging allows for the noninvasive detection of differences in blood flow that are associated with neuronal activation, with high temporal and spatial resolution. We report the case of a 6-year-old boy who underwent clinical functional MR imaging for presurgical planning of language lateralization because of a temporal-lobe lesion (Fig 1). The purpose of the study was to assess a possible interference with language-related areas with the surgical approach. The same listening-to-stories task was performed with the patient awake and after he had fallen asleep. The study revealed strong activation in similar language areas, indicating language processing during natural sleep. The detected pattern of bilateral activation (as assessed by calculating a weighted laterality index [5]) is in accordance with the high degree of variability in this age group (Fig 2).
Both images reveal remarkably similar patterns of neuronal activation, indicating that the receptive processing of heard speech does not differ fundamentally between the awake and sleeping states in this child. Similar results were seen in a combined EEG–functional MR study in adults, in which, as in our subject, only the language component of a passive listening task activated language-related brain areas (6). This case is interesting, because the child performed the same (passive) task while awake and during natural sleep; this procedure allowed us to rule out the effects of sedative agents. Although functional MR imaging has been conducted in young children under sedation (1), sedative agents have been shown to have a notable effect on the blood oxygen level–dependent (BOLD) response (7); this finding limits the conclusions to be drawn from such studies (2). Despite this advantage, however, the comparability of the two studies conducted in this child is still hampered by the involuntary motion on the first image that was not present on the second image.
Because this finding was a chance observation, no EEG recordings were available to assess the sleep stages. However, the child was asleep immediately before and after the image acquisition, and actually, he did not wake up until he was taken out of the imager several minutes after the completion of this study. Interestingly, when the pixel intensity–time courses of receptive and expressive language areas (traditionally known as the Wernicke and Broca areas [8]) are analyzed, they seem to differ substantially, with only the receptive language areas displaying a constant and robust correlation with the reference function (Fig 3). Activation in expressive areas shows a more fluctuating course and becomes robust only in the second half of the 5½-minute imaging session, whereas receptive brain areas show almost no fluctuation. This effect can be speculated to reflect different sleep stages, which is in accordance with a recent observation of a notable effect of the sleep stage on the magnitude of the BOLD response (9). In addition to a global influence, our data suggest a differential effect of different sleep stages on these areas that could be due to either anatomic or functional differences. Continuous EEG recordings would have been necessary to verify this effect.
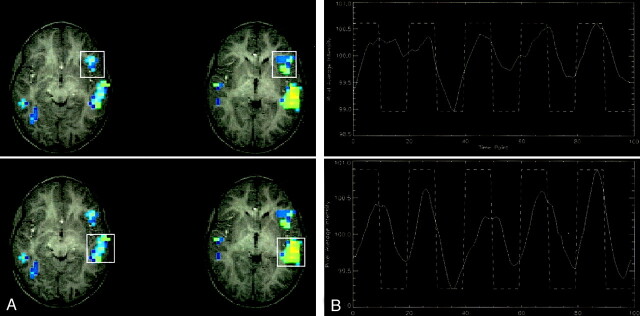
Fig 3.
Functional MR data obtained while patient was asleep.
A, Activation patterns observed during the sleeping study show activation in the expressive (top) and receptive (bottom) language areas.
B, Pixel intensity and time courses correlated with the reference function. Note the difference in responses between the expressive (top) and receptive (bottom) areas during the first three blocks. This finding suggests a regionally different response to the auditory stimulus.
Conclusion
Our observations have two major implications: First, they demonstrate that language processing during sleep is not an exclusive function of the mature brain but that it can already be detected in young children in whom language functions are still developing (5). Our findings also suggest differing effects of sleep stages on distinct brain areas. Second, by showing that language processing does occur during natural sleep, these observations indicate that this central higher brain function is amenable to functional imaging studies, even in young children in the sleep state. If natural sleep can be achieved, the confounding effects of sedative agents can thus be avoided, and robust motion-free cortical activation can still be achieved.
Acknowledgments
We would like to thank John S. Myseros, MD, and Douglas F. Rose, MD, for referring their patient.
References
- 1.Altman NR, Bernal B. Brain activation in sedated children: auditory and visual functional MR imaging. Radiology 2001;221:56–63 [DOI] [PubMed] [Google Scholar]
- 2.Miki A, Liu GT, Fletcher DW, Hunter JV, Haselgrove JC.Ocular dominance in anterior visual cortex in a child demonstrated by the use of fMRI. Pediatr Neurol. 2001;24:232–234 [DOI] [PubMed] [Google Scholar]
- 3.Stickgold R. Watching the sleeping brain watch us: sensory processing during sleep. Trends Neurosci 2001;24:307–309 [DOI] [PubMed] [Google Scholar]
- 4.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging 2001;20:535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland SK, Plante E, Weber Byars A, et al. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage 2001;14:837–843 [DOI] [PubMed] [Google Scholar]
- 6.Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron 2000;28:991–999 [DOI] [PubMed] [Google Scholar]
- 7.Martin E, Thiel T, Joeri P, et al. Effect of pentobarbital on visual processing in man. Hum Brain Mapp 2000;10:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 1997;17:353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czisch M, Wetter TC, Kaufmann C, Pollmacher T, Holsboer F, Auer DP. Altered processing of acoustic stimuli during sleep: reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimage 2002;16:251–258 [DOI] [PubMed] [Google Scholar]