Figure 1. Guanylate cyclases and natriuretic peptide receptor C.
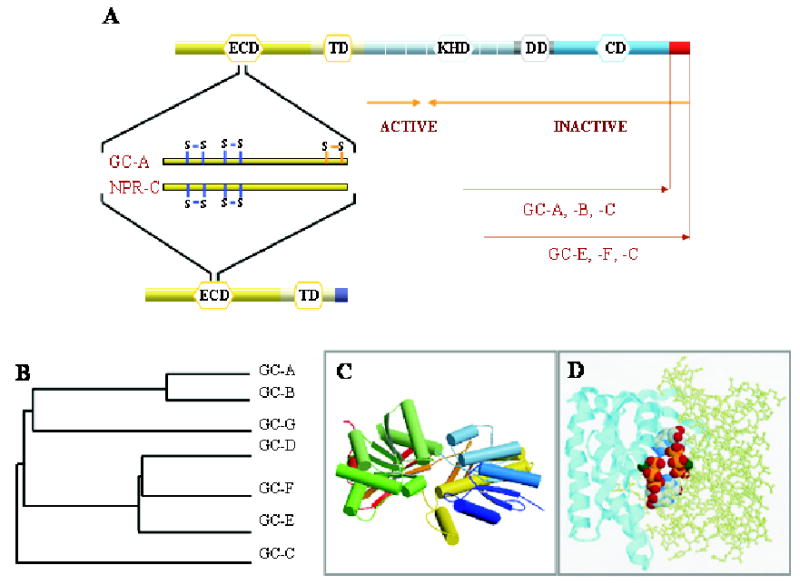
A. Topological representation of GC domains and NPR-C. ECD (extracellular domain), TM (transmembrane domain), KHD (kinase-homology domain), DD (dimerization domain), and CD (catalytic domain) are indicated. Blue lines indicates the positions of disulfide bridges as inferred from crystallographic studies in the ECD, and orange lines mark one disulfide bridge identified based on biochemical studies. Orange arrows mark the minimal truncated GC-E sequence needed for GC activity. The unique C-terminal fragment present in GC-E, -F and -C is shown as a red square. B. A phylogenetic tree calculated from the amino-acid sequences of mammalian GC is represented as the function of similarity between GCs. Analysis was performed using ClustalW program. C. Three-dimensional model of the ECD of NPR-C receptor (PDB ID: 1JDN) (He et al., 2001). Arrows represent β-strands, cylinders are α-helices. The figure was drawn by using Molscript (Kraulis, 1991) and Roster3d (Merritt & Bacon, 1997) programs. D. Model of the CD from GC-E (1AWL) (Tucker et al., 1998). Two subunits are shown in two different representation styles and two GTP molecules are shown in the default atom colors. Note that the active site is composed of residues from both subunits, and two active sites are present for the dimer. The figure was generated using the RasMol (Sayle & Milner-White, 1995).
