Figure 2. Phototransduction.
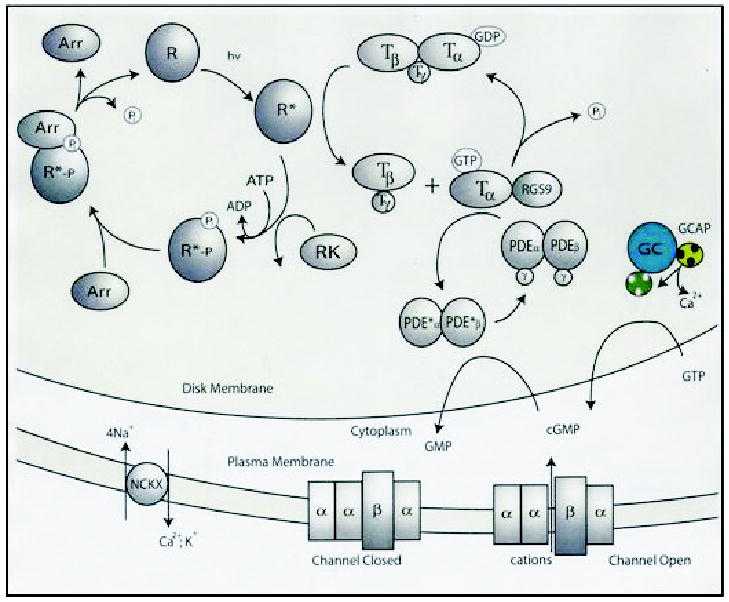
Absorption of light by rhodopsin (R) leads to transient conformation change and formation of the active form of this receptor, Meta II (or R*). In the dark, trimeric G-protein transducin (T) has bound GDP, which is replaced by GTP upon interaction with Meta II, and in turn transducin splits into α-subunit-GTP and βγ-subunits. The α-subunit-GTP activates PDE, which hydrolyses cGMP. Lowering [cGMP] causes closure of the cGMP-gated cation channel and hyperpolarization of the plasma membrane. Lower conductance of the cation through the channel also lowers [Ca2+], because the ion is removed continuously by the Na+/Ca2+-K+ exchanger. Dissociation of Ca2+ from GCAP1 leads to a conformation change in this Ca2+-binding protein and activation of photoreceptors GCs (GC-GCAP shown in colors) and enhanced synthesis of cGMP. The restoration of the dark conditions requires inactivation of Meta II, hydrolysis of GTP by α-subunit of transducin, and inactivation of PDE (for details see Palczewski et al., 2000; Polans et al., 1996). The RGS9 complex consists of RGS9 protein, β5-subunit of G-protein and a membrane anchor, R9AP protein. Abbreviations used: R, rhodopsin; R*, Meta II (photoactivated rhodopsin); RGS, regulator of G-protein signaling; RK, rhodopsin kinase; Arr, arrestin; T, transducin; NCKX, Na+/Ca2+-K+ exchanger.
