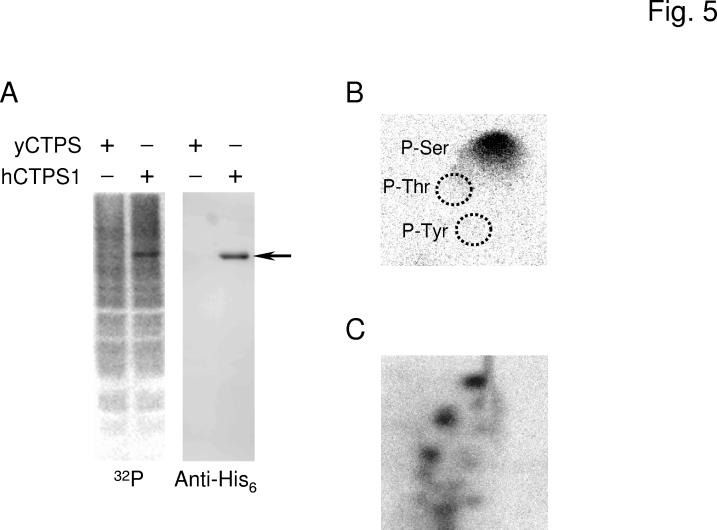
Fig. 5.
Phosphorylation of human CTP synthetase 1 in S. cerevisiae. Panel A, the ura7Δ ura8Δ mutant bearing plasmids with the yeast URA7 gene (strain GHY52) or the human CTPS1 gene (strain GHY55) was grown in 50 ml of growth medium to the exponential phase of growth. Cells were harvested, resuspended in 5 ml of fresh medium containing 32Pi (0.25 mCi/ml), and incubated for 3 h. Cell extracts were prepared from the labeled cells and incubated with Ni2+-NTA resin to bind the His6-tagged human CTP synthetase 1. Proteins bound to the Ni2+-NTA resin were eluted with SDS-PAGE treatment buffer, followed by electrophoresis and transfer to PVDF membrane. The membrane was subjected to phosphorimaging (left) followed by immunoblot analysis using anti-His6 antibodies (right). The arrow indicates the position of human CTP synthetase 1 (hCTPS1). Panel B, a PVDF membrane slice containing 32P-labeled human CTP synthetase 1 was hydrolyzed with 6N HCl for 90 min at 110 °C, and the hydrolysate was separated by 2-dimensional electrophoresis. The positions of the standard phosphoamino acids phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr) are indicated in the figure. Panel C, a PVDF membrane slice containing 32P-labeled human CTP synthetase 1 was digested with trypsin. The resulting peptides were separated on cellulose thin layer plates by electrophoresis (from left to right) in the first dimension and by chromatography (from bottom to top) in the second dimension. The data shown in the three panels were representative of two independent experiments.