Abstract
Leukocyte adhesion deficiency (LAD)–1, a primary immunodeficiency disease caused by molecular defects in the leukocyte integrin CD18 molecule, is characterized by recurrent, life-threatening bacterial infections. Myeloablative hematopoietic stem cell transplantation is the only curative treatment for LAD-1. Recently, canine LAD (CLAD) has been shown to be a valuable animal model for the preclinical testing of nonmyeloablative transplantation regimens for the treatment of children with LAD-1. To develop new allogeneic transplantation approaches for LAD-1, we assessed a nonmyeloablative conditioning regimen consisting of busulfan as a single agent before matched littermate allogeneic bone marrow transplantation in CLAD. Three CLAD dogs received busulfan 10 mg/kg intravenously before infusion of matched littermate bone marrow, and all dogs received posttransplantation immunosuppression with cyclosporin A and mycophenolate mofetil. Initially, all 3 dogs became mixed chimeras, and levels of donor chimerism sufficient to reverse the CLAD phenotype persisted in 2 animals. The third dog maintained donor microchimerism with an attenuated CLAD phenotype. These 3 dogs have all been followed up for at least 1 year after transplantation. These results indicate that a nonmyeloablative conditioning regimen with chemotherapy alone is capable of generating stable mixed chimerism and reversal of the disease phenotype in CLAD.
Keywords: Canine leukocyte adhesion deficiency, Nonmyeloablative conditioning, Busulfan, Bone marrow transplantation
INTRODUCTION
Leukocyte adhesion deficiency (LAD)–1 is a genetic immunodeficiency disease that results from heterogeneous mutations in the leukocyte integrin CD18 molecule [1,2]. The inability of LAD-1 leukocytes to adhere to the vessel wall and migrate to sites of infection as a result of CD18 mutations accounts for the disease phenotype, which consists of episodes of life-threatening bacterial infection, leukocytosis with mature neutrophilia, advanced periodontal disease, and markedly impaired wound healing. Depending on the level of expression of CD18, LAD-1 can be categorized into moderate and severe phenotypes [3]. Children with the severe-deficiency phenotype express less than 1% of normal levels of CD18 and typically develop lethal bacterial infections within the first years of life. Children with the moderate phenotype have higher levels of CD18 expression in their cells (1%-10% of normal levels) and survive into the second or third decade of life [3].
Allogeneic hematopoietic stem cell transplantation after myeloablative conditioning is currently the only definitive therapy for LAD-1 [4,5]. Historically, the goal of myeloablative conditioning in this setting is to achieve 100% donor chimerism. However, it seems that complete donor chimerism is not required for correction of the clinical phenotype in LAD-1. Analysis of a small number of LAD-1 patients who developed mixed donor chimerism after a myeloablative conditioning regimen indicates that the mixed chimeric state resulted in reversal of the disease phenotype [5]. These results suggest that a nonmyeloablative transplant regimen used to achieve mixed chimerism may be effective in LAD-1, thus sparing the child with LAD-1 the regimen-related toxicity associated with myeloablation before transplantation [5].
We recently established a colony of mixed-breed dogs with canine LAD (CLAD) to test new transplantation approaches to this disease. CLAD represents the canine equivalent of the severe-deficiency phenotype of LAD-1 in children [6]. Puppies with CLAD experience recurrent life-threatening infections that typically culminate in death by 6 months of age [7,8]. CLAD is due to a single DNA point mutation (G107C) and amino acid substitution (C36S) in the leukocyte integrin CD18 subunit; this mutation results in the inability to express the CD18 subunit on the leukocyte surface [9]. In previous studies, we established that a nonmyeloablative transplantation regimen consisting of 200 cGy of total body irradiation (TBI), infusion of matched littermate bone marrow, and a short posttransplantation immunosuppressive regimen with cyclosporin A (CsA) and mycophenolate mofetil (MMF) resulted in stable mixed donor-host chimerism and reversal of the CLAD phenotype [10].
This study assessed whether single-agent intravenous busulfan could replace TBI in the nonmyeloablative conditioning regimen. Two of the 3 CLAD dogs treated with this regimen achieved levels of donor chimerism that reversed the disease phenotype. The third dog has donor microchimerism with attenuation of the severe CLAD phenotype and remains alive more than 2 years after transplantation.
MATERIALS AND METHODS
Dogs
All procedures involving animals in this study were approved by the Animal Care and Use Committee of the National Cancer Institute (Bethesda, MD). These studies were conducted in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals published by the National Research Council of the National Academy of Sciences. Animals were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, either on the campus of the National Institutes of Health in Bethesda, MD, or at the National Institutes of Health satellite facility in Poolesville, MD.
All animals were immunized against common canine viral diseases and were monitored at least twice a day by the veterinary care staff. The temperature, pulse, and respirations were recorded for each dog from 4 weeks of age until at least 4 months after transplantation. A veterinarian examined all dogs showing signs of illness and prescribed treatment as clinically indicated. Fevers were managed with broad-spectrum antibiotics according to the results of culture and physical examination, and pain was treated with appropriate narcotic or nonnarcotic analgesics. Matched litter-mate donor-recipient pairs were identified by using highly polymorphic microsatellite markers near the dog leukocyte antigen (DLA) loci in the major histocompatibility complex, as previously described [8,11].
CLAD Phenotype Identification
CLAD-affected dogs were identified by flow cytometry of peripheral blood leukocytes by using anti-CD18 monoclonal antibodies (DakoCytomation, Carpinteria, CA) labeled with fluorescein isothiocyanate to demonstrate the absence of CD18 on the cell surface, as previously described [8]. This anti-human CD18 antibody cross-reacts with canine CD18 [8,12].
Bone Marrow Harvests
Bone marrow was harvested from donors, and the CD34+ cells were isolated as previously described [13,14]. Briefly, marrow was aspirated from the bilateral humeri, femora, and tibiae through Jamshidi needles (Cardinal Health, Dublin, OH) while dogs were under general anesthesia. Up to 15 mL/kg donor weight of marrow was aspirated into heparinized syringes and filtered by using a commercial bone marrow collection kit according to the manufacturer's instructions (Baxter Healthcare Corporation, Deer-field, IL). CD34+ cells were quantified from an aliquot of marrow by using phycoerythrin-conjugated monoclonal antibody 1H6 directed against canine CD34 (BDPharmingen, San Diego, CA) [15].
Transplantation Conditioning, Bone Marrow Infusion, and Posttransplantation Immunosuppression
The 3 dogs that received matched littermate bone marrow infusions underwent transplantation before 3 months of age. Pretransplantation conditioning consisted of 10 mg/kg busulfan (Busulfex; ESP Pharma, Edison, NJ) administered intravenously over 1 hour on day −2. This dose of busulfan has previously been shown to be nonmyeloablative in healthy dogs [16]. CLAD dogs were pretreated with 2 mg/kg diphenhydramine (Elkins-Sinn, Inc., Cherry Hill, NJ) and 0.22 mg/kg ondansetron (Zofran; GlaxoSmithKline, Research Triangle Park, NC), both given intravenously to reduce the incidence of emesis. On the day of transplantation, designated day 0, fresh unmanipulated whole bone marrow was infused intravenously over 30 minutes.
Posttransplantation immunosuppression consisted of CsA (Sandimmune; Novartis, East Hanover, NJ) and MMF (CellCept; Roche, Nutley, NJ). Recipient dogs received 15 mg/kg CsA orally twice daily starting on day −1 and continuing until day +35. From day +36 to day +65, CsA was given orally twice daily at 7.5 mg/kg. MMF was given orally at 10 mg/kg twice daily from day 0 until day 28.
Clinical Assessment and Posttransplantation Care
After transplantation, dogs were monitored clinically twice a day for behavior, activity level, vital signs, and weight. A veterinarian examined all dogs showing signs of illness and prescribed treatment as clinically indicated. Fevers were managed with broad-spectrum antibiotics according to the results of culture and physical examination. Pain was treated with appropriate narcotic or nonnarcotic analgesics. Complete blood counts and blood chemistry tests were evaluated weekly from weeks 2 to 6, every two weeks from weeks 7 through 16, monthly from week 17 to week 52, and every 3 months thereafter (or as clinically indicated).
Chimerism Analysis after Transplantation
Donor chimerism was quantified after transplantation by using flow cytometry to detect donor CD18+ leukocytes, as previously described [13]. Flow cytometric chimerism assays were performed weekly from weeks 2 to 6, every two weeks from weeks 7 through 16, monthly from week 17 to week 52, and every 3 months thereafter.
Statistics
Survival curves were calculated according to the method of Kaplan and Meier [17]. The 2-tailed log-rank test was used as a test of significance.
RESULTS
Clinical Course of Control CLAD Dogs
CLAD dogs develop a constellation of clinical manifestations of the disease starting at approximately 6 weeks of age [7,8]. Examples of these findings in our colony are shown in Figure 1. Craniomandibular osteopathy and hypertrophic osteodystrophy (HOD) of the extremities are noninfectious, inflammatory proliferations of the bone of unknown etiology (Figure 1A, B, and D). The gingivitis (Figure 1C) and nonhealing wounds (not shown) in CLAD dogs mirror findings in young children with LAD-1 [18].
Figure 1.
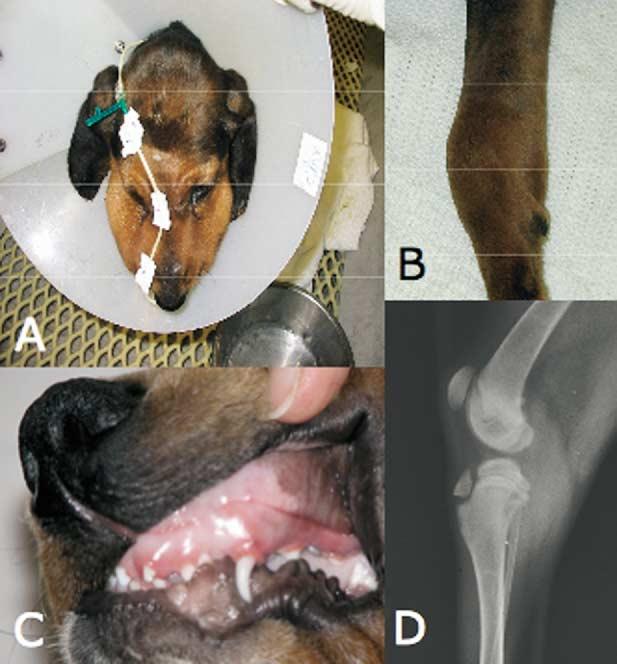
Clinical manifestations of CLAD. A, Hypertrophy of the muzzle due to craniomandibular osteopathy (CMO). The nasojejunal tube is used for total enteral nutrition and is necessitated by the dog's inability to open its jaws fully because of CMO. The Elizabethan collar is to prevent the dog's removing an intravenous catheter necessary for the administration of antibiotics. B, Foreleg of a CLAD dog with HOD and carpal swelling. C, Gingivitis, likely due to a combination of CMO and oral infection. D, Forelimb radiograph of a dog with HOD. The double physis sign, pathognomonic for HOD, is evident as lucent bands proximal to the femoral and distal to the tibial epiphyses.
The 3 CLAD dogs in our colony that lacked a matched littermate were euthanized after several months of life because of intractable symptoms of CLAD. Two of the CLAD dogs (Poliwag and Velma) were euthanized at 2 and 4 months of age, respectively, because of severe HOD and craniomandibular osteopathy. The third dog (Cayenne) was euthanized at 6 months of age because of severe recurrent skin infections associated with resistant microorganisms. Necropsy reports of the 3 dogs showed lymphadenopathy, splenomegaly, HOD, marrow hyperplasia, multiple dermal abscesses, tracheal ulcers, congestive neutrophilic hepatitis, jejunal abscess, or a combination of these (data not shown).
Bone Marrow Transplantation
Three CLAD dogs (Frodo, Billy, and Vixen) received transplants from matched littermate donors before 3 months of age. All 3 were symptomatic with fever, infection, and/or HOD before transplantation. All 3 dogs received a nonmyeloablative conditioning regimen consisting of 10 mg/kg busulfan administered intravenously over 1 hour and given 48 hours before transplantation (Figure 2). Details of the cell products infused are presented in Table 1. All 3 animals received >5 × 106 bone marrow CD34+ cells per kilogram (Table 1).
Figure 2.
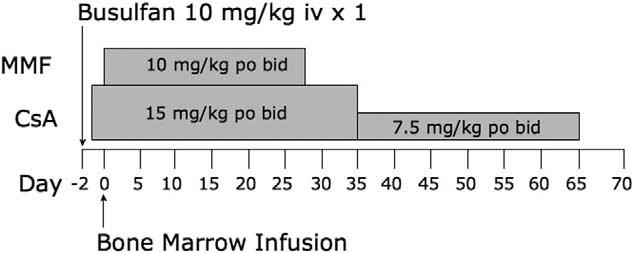
Nonmyeloablative transplantation regimen for CLAD using busulfan. Three CLAD pups received busulfan 10 mg/kg intravenously on day −2. On day 0, bone marrow was harvested from matched littermate donors and infused intravenously into the CLAD pups. Dogs received immunosuppression with MMF 10 mg/kg orally twice daily for 28 days and with CsA 15 mg/kg orally twice daily for 35 days, and then they received 7.5 mg/kg orally twice daily for 30 days. The peritransplantation day is shown on the x-axis. po indicates orally; iv, intravenously; bid, twice daily.
Table 1.
Details of CD34+ Cell Transplantations and Clinical Outcomes in CLAD Dogs after Busulfan Conditioning
| % of CD18+ Leukocytes at Last Follow-Up |
||||||||
|---|---|---|---|---|---|---|---|---|
| Dog | Age at Transplantation (wk) | CD34+ Cells Infused (/kg) | Length of Follow-Up (mo) | Neutrophils | CD3+ Lymphocytes | Total | WBC Count at Last Follow-Up (/μL) | Clinical Status at Last Follow-Up |
| Frodo | 12 | 6.7 × 106 | 24 | 10.0% | 47.5% | 15.9% | 11 000 | Alive and well |
| Billie | 10 | 17.1 × 106 | 24 | 0.03% | 3.12% | 0.64% | 24 400 | Alive on prophylactic antibiotics |
| Vixen | 10 | 12.3 × 106 | 12 | 8.6% | 22.0% | 11.8% | 10 800 | Alive and well |
WBC indicates white blood cells.
The transplantation regimen was well tolerated by all 3 dogs, with no infusional toxicity or regimen-related morbidity. The hematologic toxicity of the transplant regimen was mild, with a leukocyte nadir (mean ± SD) of 8.8 ± 0.4 × 103 cells per microliter (canine normal leukocyte range, 4-15.5 × 103 cells per microliter; Antech Diagnostics, Lake Success, NY). Platelet and hematocrit nadirs were 41 ± 14 × 103 cells per microliter (normal range, 170-400 × 103 cells per microliter) and 28.1% ± 1.37% (normal range, 36%-60%), respectively. No dog required transfusional support.
Posttransplantation immunosuppression with CsA was continued for 2 months, and MMF was continued for 1 month (Figure 2). The major complication from immunosuppression with CsA and MMF was emesis. CsA also caused varying degrees of gingival hyperplasia in all of the dogs; it resolved after completion of immunosuppression at day 65.
Engraftment and Chimerism
All 3 CLAD dogs that received transplants initially engrafted. Two dogs, Frodo and Vixen, achieved stable mixed donor-host chimerism: Frodo displayed donor chimerism of 15.9% CD18+ donor leukocytes at 2 years after transplantation, and Vixen had 11.8% CD18+ donor leukocytes 1 year after transplantation. Although Frodo and Vixen remain stable mixed chimeras, the percentage of CD18+ donor-derived leukocytes declined between approximately day 100 and day 350 before plateauing (Figure 3). A notable feature of both Frodo's and Vixen's posttransplantation course is that the nonmyeloablative transplantation resulted in a split lymphoid-myeloid chimerism. In Frodo's case, CD3+ chimerism is nearly 3-fold greater than myeloid chimerism, with 47.5% donor CD3+ cells in the peripheral blood (Table 1).
Figure 3.
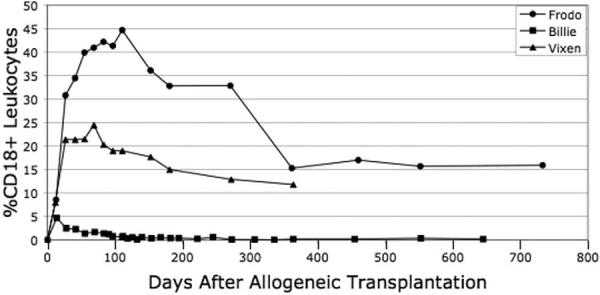
Leukocyte chimerism after transplantation. Blood was collected from each dog at the designated intervals after transplantation, and leukocytes were isolated and stained with either an anti-CD18 monoclonal antibody or an isotype control, as described in “Materials and Methods.”
The third dog, Billie, displayed initial engraftment followed by decreasing levels of CD18+ donor-derived leukocytes over the 2 months after transplantation. This culminated in a state of donor microchimerism, with 0.64% of the peripheral blood leukocytes of donor origin 24 months after transplantation (Table 1; Figure 3).
Clinical Outcomes of Transplantations and Extended Follow-up
Frodo and Billie are now 2 years after transplantation, and Vixen is 1 year after transplantation (Table 1). Frodo and Vixen are clinically indistinguishable from unaffected dogs (Figure 4). They are housed in standard conditions with other healthy dogs. Billie has had intermittent skin and gingival infections and receives prophylactic oral antibiotics (Figure 4). Nevertheless, this dog remains alive 2 years after transplantation, markedly exceeding the 6-month life expectancy of CLAD dogs that did not undergo transplantation in our studies and others [7,10]. None of the 3 CLAD dogs that received transplants developed acute or chronic graft-versus-host disease (GVHD) after matched littermate allotransplantation. As compared with the control dogs, dogs that underwent transplantation had significantly improved survival (Figure 5; P = .025 by 2-tailed log-rank test).
Figure 4.
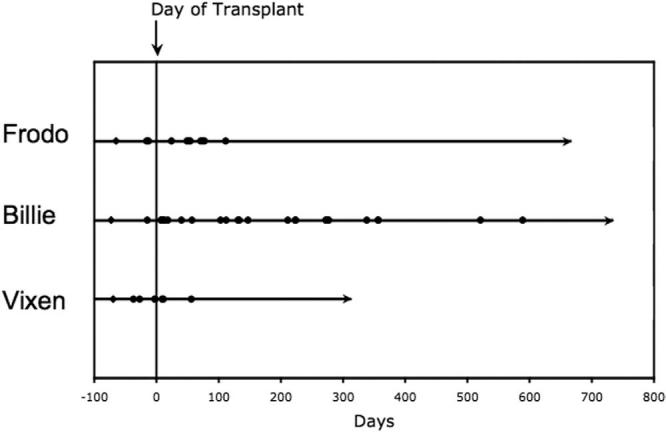
Clinical course of CLAD dogs that received transplants. Dogs were monitored clinically and treated for fever, pain, and infection as described in “Materials and Methods.” The day of transplantation is indicated by a vertical line. Days with fever ≥103°F (39.4°C) are indicated by filled circles. The day of birth is indicated by a black diamond. Last follow-up is indicated by an arrow.
Figure 5.
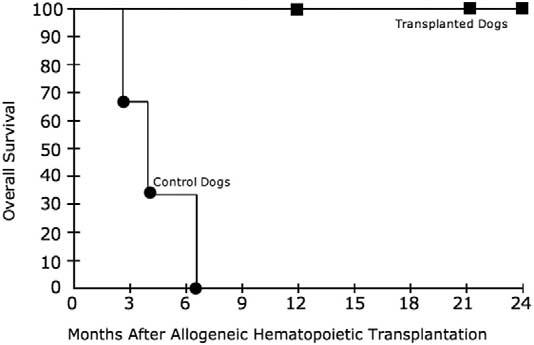
Overall survival of control CLAD dogs and those that underwent transplantation. Curves were generated according to the method of Kaplan and Meier. The upper curve represents the dogs that underwent transplantation, and the lower curve represents the control dogs. Squares represent last follow-up for living dogs. Circles represent the time of death.
There is no standard laboratory measurement of CLAD disease activity; however, the peripheral blood leukocyte count serves as a surrogate marker for CLAD [8]. Of the dogs presented here, Frodo and Vixen have resolved leukocytosis, with white blood cell counts of 11 000 and 10 800 cells per microliter at 104 and 52 weeks after transplantation, respectively, whereas Billie's total white blood cell counts have remained increased, at 24 000 cells per microliter at 97 weeks after transplantation, predominantly as a result of a mature neutrophilia. Neutrophilia is a typical finding in CLAD dogs. The clinical outcomes of the transplantations are summarized in Table 1.
DISCUSSION
We have demonstrated that a nonmyeloablative bone marrow transplantation regimen consisting of pretransplantation conditioning with busulfan, along with a brief course of posttransplantation immunosuppression with CsA and MMF, can result in reversal of the disease phenotype in CLAD. The regimen described in this article used matched littermate donors and resulted in mixed donor-host chimerism in all 3 CLAD dogs. Two of the 3 dogs displayed donor leukocyte chimerism of 10% to 15%, whereas the third dog had donor microchimerism of <1%. All 3 dogs are alive and well more than 1 year after transplantation, having markedly exceeded the 6-month life expectancy of dogs with CLAD [7]. Two dogs are clinically indistinguishable from healthy littermates, whereas the 1 dog with donor microchimerism has an attenuated CLAD phenotype resembling the moderate-deficiency phenotype of LAD-1. These data indicate that a nonmyeloablative transplantation regimen consisting of chemotherapy and immunosuppression without TBI can reverse the phenotype in CLAD.
Hematopoietic cell transplantation is the only curative therapy for LAD-1. Despite recent interest in nonmyeloablative and reduced-intensity conditioning regimens, the standard of care for conditioning before transplantation for LAD-1 remains a myeloablative regimen with its attendant toxicities [19]. In this setting, significant regimen-related toxicity and GVHD remain major causes of morbidity and mortality and limit the usefulness of transplantation [19]. In the largest series of transplantations for LAD-1 reported to date, which used a variety of myeloablative preparative regimens, 4 of 14 patients died, 5 of the 10 survivors had grade II to IV acute GVHD, and 3 of those 5 had chronic GVHD [19].
We developed the CLAD model to test nonmyeloablative transplantation regimens for the treatment of LAD-1 [8,13,14]. The CLAD model recapitulates the severe-deficiency LAD-1 phenotype, including poorly healing skin lesions, leukocytosis, immunodeficiency, gingivitis, and early death [8,20]. Moreover, this model is particularly suited to the study of transplantation for LAD-1, building on the extensive experience with dogs as a model for allogeneic transplantation in general [21]. Prior studies from our laboratory have used nonmyeloablative conditioning with 200 cGy of TBI, followed by bone marrow transplantation from DLA-identical littermates and immunosuppression with CsA and MMF, to induce stable mixed chimerism and reversal of the CLAD pheno-type [10,14].
To investigate alternatives to TBI, we explored a busulfan-based conditioning regimen before matched littermate transplantation in CLAD. Busulfan has not previously been used as a single agent in nonmyeloablative transplantation regimens in humans; however, several considerations, both practical and theoretical, led to our choice of this drug. Of the 3 alkylating agents most commonly used in pretransplantation conditioning regimens—busulfan, cyclophosphamide, and melphalan—busulfan has most often been used as a replacement for TBI and has the longest history of use in the pediatric population, which is the population most likely to undergo nonmyeloablative transplantation for LAD-1. Furthermore, busulfan has been studied in autologous marrow transplantations in healthy dogs [16]. In those studies, marrow ablation was achieved at a dose of 20 mg/kg, whereas 10 mg/kg busulfan, the dose chosen for these studies, was found to be nonmyeloablative [16]. Other agents commonly used in nonmyeloablative transplantation approaches in humans, either with or without busulfan, are fludarabine and anti–T-cell antibody preparations. Fludarabine is metabolized much more quickly in dogs than it is in humans, thus making its use impractical [22]. With respect to antibodies, there is no experience with the anti-CD52 antibody alemtuzumab in dogs. Antithymocyte or antilymphocyte serum has been used previously; however, these reagents must be custom manufactured, and the preparations are more difficult to standardize in dogs than in humans.
Because of the limitations of oral busulfan, we chose to use the recently approved intravenous formulation [23-25]. The initial experience with the intravenous formulation showed that the pharmacokinetics were more predictable than those of the oral formulation of the drug [26-28]. In a clinical setting, intravenous administration of busulfan led to consistent engraftment from related and unrelated donors.
Historically, LAD-1 patients have needed more conditioning than other patients to achieve myeloablation and facilitate engraftment. In particular, LAD-1 children have required the addition of etoposide to standard myeloablative doses of busulfan and cyclophosphamide to reliably achieve engraftment and full donor chimerism [19,29,30]. For this reason, there was concern that the dose of busulfan that allows for engraftment in humans and dogs without LAD-1 or CLAD might be insufficiently myeloablative to lead to engraftment in CLAD dogs or in LAD-1 patients because of the hyperexpanded bone marrow in both LAD-1 and CLAD. These studies were designed to address this issue in the disease-specific large-animal model of LAD-1.
Compared with ablative transplantations with or without TBI, the busulfan conditioning regimen described in this article was well tolerated. Leukopenia did not occur, nor did thrombocytopenia or anemia necessitating transfusion. There were no infections that could not be easily managed with antibiotics. There was no serious regimen-related toxicity. Long-term toxicity from busulfan was also not observed. Specifically, liver-associated enzymes have been normal at all time points when they have been assayed, except for a single asymptomatic increase of aspartate aminotransferase in Billie, and this level subsequently spontaneously returned to normal. The dogs are able to exercise to the same extent as carrier and wild-type animals, with no signs or symptoms of pulmonary toxicity. Furthermore, none of the 3 dogs developed GVHD. In addition to being easily tolerated, this regimen was effective, resulting in reversal of the CLAD phenotype in 2 dogs and its attenuation in the third animal.
One area of initial concern in our treated dogs was the decline in donor chimerism in Frodo and Vixen between 100 and 350 days after transplantation. However, both dogs' level of donor chimerism subsequently plateaued. This apparent decrease in chimerism is consistent with delayed recovery of normal host hematopoietic stem cells after treatment with busulfan, rather than delayed graft rejection [31,32].
Partial chimerism is typically seen as problematic in studies of nonmyeloablative transplantation, such that donor lymphocyte infusions have been used to convert to full donor chimerism even if the disease for which stem cell transplantation was indicated is no longer evident [33]. These studies typically have been in the setting of neoplastic disease, where one can expect—and where most experience has shown—that any residual host hematopoiesis will lead to disease recurrence [34-36]. This is not the case in primary immunodeficiency. LAD-1 patients with partial donor chimerism after allogeneic transplantation have reversal of the disease phenotype; this argues against the need for full donor chimerism, with the attendant increased risk of GVHD, in this setting [19]. Previous reports from our laboratory have examined the minimal level of donor chimerism necessary to reverse the disease phenotype in CLAD [14]. Two dogs with only 10% CD18+ white blood cells had complete reversal of the CLAD phenotype. Even given these findings, it is still somewhat surprising that Billie is able to survive with less than 1% CD18+ cells only on prophylactic antibiotics. The most likely explanation is that the CD18+ neutrophils in Billie selectively migrate into tissues, and, thus, measurement of CD18+ neutrophils in the peripheral blood may underestimate her tissue neutrophils. This likely explains her benign clinical course. Also, because Billie has less than 1% CD18+ cells in her marrow, it is accurate to refer to Billie as having less than 1% donor chimerism.
The mixed chimerism in the 3 dogs described in this study may be one factor in the animals' absence of GVHD. There is evidence that mixed chimerism is tolerogenic, and thus our dogs' mixed chimerism may explain the lack of GVHD [37-39].
One limitation in the interpretation of our data is the inbred nature of our colony. Considerable measures were taken at the outset to obtain genetic diversity in our colony through breeding with unrelated wild-type dogs at several early points in the development of the colony [8]. Nevertheless, some increased degree of homozygosity for minor histocompatibility antigens in excess of that seen in random outbred carriers inherently exists in an inbred colony, despite efforts to maintain genetic diversity. Our observations of engraftment might be less consistent in large animals from less inbred colonies or in humans. Further studies may be required in healthy dogs (more outbred) that are not part of our CLAD colony.
In summary, these studies indicate that a preparative regimen of 10 mg/kg busulfan given as a single intravenous infusion allows engraftment of DLA-identical matched littermate bone marrow in dogs with CLAD. Levels of engraftment achievable with this method are sufficient to attenuate or reverse the severe CLAD phenotype. Because this regimen did not lead to sufficient levels of chimerism to completely reverse the CLAD phenotype in all 3 animals, additional agents may be required to translate this success into clinical application in settings where predictably higher levels of donor chimerism are required and where the degree of matching is more disparate than in the canine model. Despite these caveats, the nonmyeloablative regimen described in this article results in mixed chimerism and reversal or attenuation of the disease phenotype in CLAD, thus suggesting that a similar approach may be clinically applicable in children with LAD-1.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
REFERENCES
- 1.Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogenous mutations in the β subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50:193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA, Thompson WS, Miller LJ, Schmalstieg FC, Anderson DC. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984;160:1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 4.Le Diest F, Blanche S, Keable H, et al. Successful HLA non-identical bone marrow transplantation in three patients with the leukocyte adhesion deficiency. Blood. 1989;74:512–516. [PubMed] [Google Scholar]
- 5.Thomas C, Le Deist F, Cavazzana-Calvo M, et al. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood. 1995;86:1629–1635. [PubMed] [Google Scholar]
- 6.Renshaw HW, Chatburn C, Bryan GM, Bartsch RC, Davis WC. Canine granulocytopathy syndrome: neutrophil dysfunction in a dog with recurrent infections. J Am Vet Med Assoc. 1975;166:443–447. [PubMed] [Google Scholar]
- 7.Trowald-Wigh G, Ekman S, Hansson K, Hedhammar Å , Hård af Segerstad C. Clinical, radiological and pathological features of 12 Irish setters with canine leucocyte adhesion deficiency. J Small Anim Pract. 2000;41:211–217. doi: 10.1111/j.1748-5827.2000.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 8.Creevy KE, Bauer TR, Jr, Tuschong LM, et al. Canine leukocyte adhesion deficiency colony for investigation of novel hematopoietic therapies. Vet Immunol Immunopathol. 2003;94:11–22. doi: 10.1016/s0165-2427(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 9.Kijas JMH, Bauer TR, Jr, Gäfvert S, et al. A missense mutation in the β-2 integrin gene (ITGB2) causes canine leukocyte adhesion deficiency. Genomics. 1999;61:101–107. doi: 10.1006/geno.1999.5948. [DOI] [PubMed] [Google Scholar]
- 10.Bauer TR, Jr, Gu YC, Tuschong LM, et al. Nonmyeloablative hematopoietic stem cell transplantation corrects the disease phenotype in the canine model of leukocyte adhesion deficiency. Exp Hematol. 2005;33:706–712. doi: 10.1016/j.exphem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JL, Burnett RC, DeRose SA, et al. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen CN, Aasted B, Broe MK, Petersen JL. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Vet Immunol Immunopathol. 1993;39:461–466. doi: 10.1016/0165-2427(93)90075-f. [DOI] [PubMed] [Google Scholar]
- 13.Creevy KE, Bauer TR, Jr, Tuschong LM, et al. Mixed chimeric hematopoietic stem cell transplant reverses the disease phenotype in canine leukocyte adhesion deficiency. Vet Immunol Immunopathol. 2003;95:113–121. doi: 10.1016/s0165-2427(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 14.Bauer TR, Jr, Creevy KE, Gu YC, et al. Very low levels of donor CD18+ neutrophils following allogeneic hematopoietic stem cell transplantation reverse the disease phenotype in canine leukocyte adhesion deficiency. Blood. 2004;103:3582–3589. doi: 10.1182/blood-2003-11-4008. [DOI] [PubMed] [Google Scholar]
- 15.Bruno B, Nash RA, Wallace PM, et al. CD34+ selected bone marrow grafts are radioprotective and establish mixed chimerism in dogs given high dose total body irradiation. Transplantation. 1999;68:338–344. doi: 10.1097/00007890-199908150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Deeg HJ, Schuler US, Shulman H, et al. Myeloablation by intravenous busulfan and hematopoietic reconstitution with autologous marrow in a canine model. Biol Blood Marrow Transplant. 1999;5:316–321. doi: 10.1016/s1083-8791(99)70007-8. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Anderson DC, Schmalsteig FC, Finegold MJ, et al. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985;152:668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- 19.Thomas C, Le Deist F, Cavazzana-Calvo M, et al. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood. 1995;86:1629–1635. [PubMed] [Google Scholar]
- 20.Bauer TR, Jr, Gu YC, Creevy KE, et al. Leukocyte adhesion deficiency in children and Irish setter dogs. Pediatr Res. 2004;55:363–367. doi: 10.1203/01.PDR.0000111287.74989.1B. [DOI] [PubMed] [Google Scholar]
- 21.Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11–15. [PubMed] [Google Scholar]
- 22.El Dareer SM, Struck RF, Tillery KF, et al. Disposition of 9-beta-D-arabinofuranosyl-2-fluoroadenine in mice, dogs, and monkeys. Drug Metab Dispos. 1980;8:60–63. [PubMed] [Google Scholar]
- 23.Tran H, Petropoulos D, Worth L, et al. Pharmacokinetics and individualized dose adjustment of intravenous busulfan in children with advanced hematologic malignancies undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:805–812. doi: 10.1016/j.bbmt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Zwaveling J, Bredius RG, Cremers SC, et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant. 2005;35:17–23. doi: 10.1038/sj.bmt.1704707. [DOI] [PubMed] [Google Scholar]
- 25.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 26.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 27.Andersson BS, Kashyap A, Gian V, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant. 2002;8:145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- 28.Andersson BS, Kashyap A, Couriel D, et al. Intravenous busulfan in pretransplant chemotherapy: bioavailability and patient benefit. Biol Blood Marrow Transplant. 2003;9:722–724. doi: 10.1016/j.bbmt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Farinha NJ, Duval M, Wagner E, et al. Unrelated bone marrow transplantation for leukocyte adhesion deficiency. Bone Marrow Transplant. 2002;30:979–981. doi: 10.1038/sj.bmt.1703719. [DOI] [PubMed] [Google Scholar]
- 30.Mancias C, Infante AJ, Kamani NR. Matched unrelated donor bone marrow transplantation in leukocyte adhesion deficiency. Bone Marrow Transplant. 1999;24:1261–1263. doi: 10.1038/sj.bmt.1702049. [DOI] [PubMed] [Google Scholar]
- 31.Kuramoto K, Follman D, Hematti P, et al. The impact of low-dose busulfan on clonal dynamics in nonhuman primates. Blood. 2004;104:1273–1280. doi: 10.1182/blood-2003-08-2935. [DOI] [PubMed] [Google Scholar]
- 32.Laukkanen MO, Kuramoto K, Calmels B, et al. Low-dose total body irradiation causes clonal fluctuation of primate hematopoietic stem and progenitor cells. Blood. 2005;105:1010–1015. doi: 10.1182/blood-2004-04-1498. [DOI] [PubMed] [Google Scholar]
- 33.Daly A, McAfee S, Dey B, et al. Nonmyeloablative bone marrow transplantation: infectious complications in 65 recipients of HLA-identical and mismatched transplants. Biol Blood Marrow Transplant. 2003;9:373–382. doi: 10.1016/s1083-8791(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 34.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for preemptive immunotherapy? J Clin Oncol. 2004;22:1696–1705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 35.Juliusson G, Karlsson K, Malm C, et al. Adjusted conditioning for allogeneic transplantation in a single center setting: mixed chimerism heralds relapse. Leuk Lymphoma. 2003;44:669–679. doi: 10.1080/1042819031000063372. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Aviles F, Urbano-Ispizua A, Aymerich M, et al. Serial quantification of lymphoid and myeloid mixed chimerism using multiplex PCR amplification of short tandem repeat-markers predicts graft rejection and relapse, respectively, after allogeneic transplantation of CD34+ selected cells from peripheral blood. Leukemia. 2003;17:613–620. doi: 10.1038/sj.leu.2402854. [DOI] [PubMed] [Google Scholar]
- 37.Mattsson J, Uzunel M, Remberger M, Ringden O. T cell mixed chimerism is significantly correlated to a decreased risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2001;71:433–439. doi: 10.1097/00007890-200102150-00017. [DOI] [PubMed] [Google Scholar]
- 38.Huss R, Deeg HJ, Gooley T, et al. Effect of mixed chimerism on graft-versus-host disease, disease recurrence and survival after HLA-identical marrow transplantation for aplastic anemia or chronic myelogenous leukemia. Bone Marrow Transplant. 1996;18:767–776. [PubMed] [Google Scholar]
- 39.McSweeney PA, Storb R. Mixed chimerism: preclinical studies and clinical applications. Biol Blood Marrow Transplant. 1999;5:192–203. doi: 10.1053/bbmt.1999.v5.pm10465099. [DOI] [PubMed] [Google Scholar]


