Figure 2.
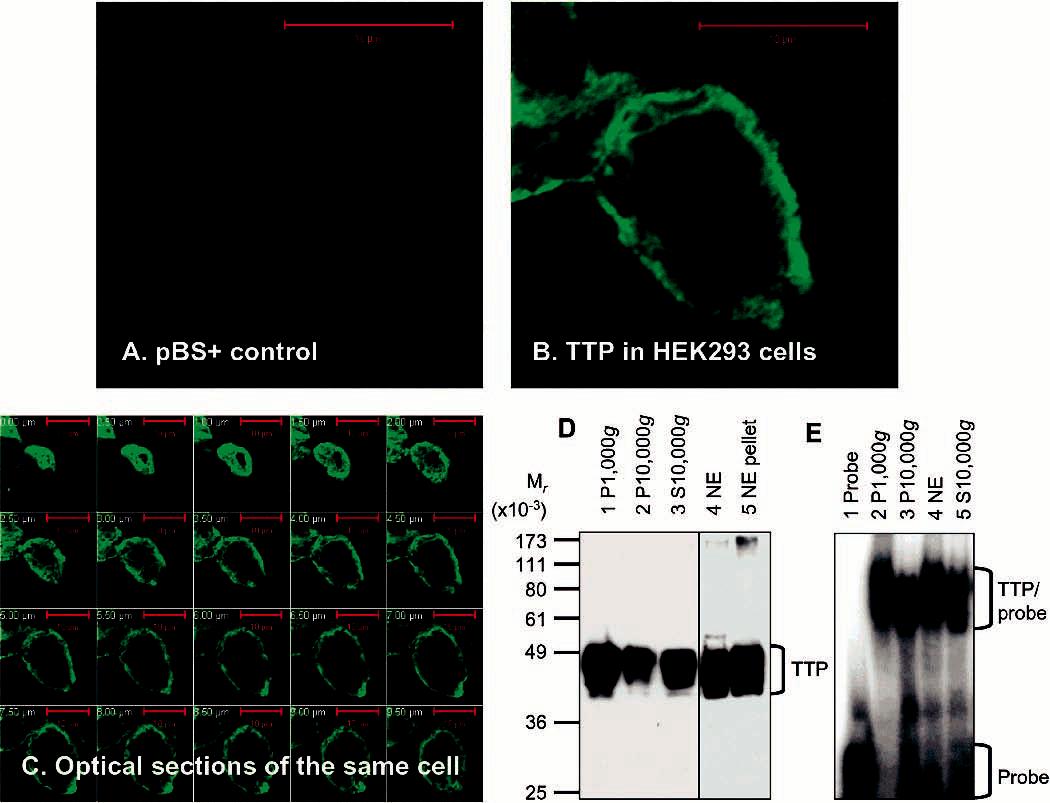
Expression and localization of active TTP in transfected human cells. (A–C) Expression and localization of TTP by immunocytochemistry. HEK293 cells were transfected with pBS+ control plasmid (A) and pHis-hTTP plasmid (B and C). The cells were immunostained with the anti-MBP-hTTP serum (1:5000 dilution) and labeled with goat anti-rabbit Alexa Fluor 488 (1:1000 dilution). Immunofluorescence was recorded with a confocal microscope as either a single image (A and B) or serial images of optical sections of a single cell with a 0.5 μm interval (C). (D) Expression and localization of TTP by immunoblotting. HEK293 cells were transfected with pHis-hTTP. The lysate was centrifuged at 1000g, resulting in S1000g and P1000g. S1000g was further centrifuged at 10000g, resulting in S10000g and P10000g. P1000g was extracted with a buffer containing 0.45 M KCl and centrifuged at 10000g, resulting in the 10000g supernatant (nucleic extract, NE) and the 10000g pellet (NE pellet). Proteins (8 μL) were separated by 10% SDS–PAGE, transferred onto a nitrocellulose membrane, and detected with the anti-MBP–hTTP serum. The position of TTP is indicated. The S10000g lane was loaded with proteins extracted from 10% of the cells used for other samples. (E) ARE binding activity by GMSA. The ARE binding activity was assayed by using proteins prepared as described for panel D. Each assay used 1 μL of each fraction. The positions of TTP–probe complexes and free probes are indicated.
