Figure 4.
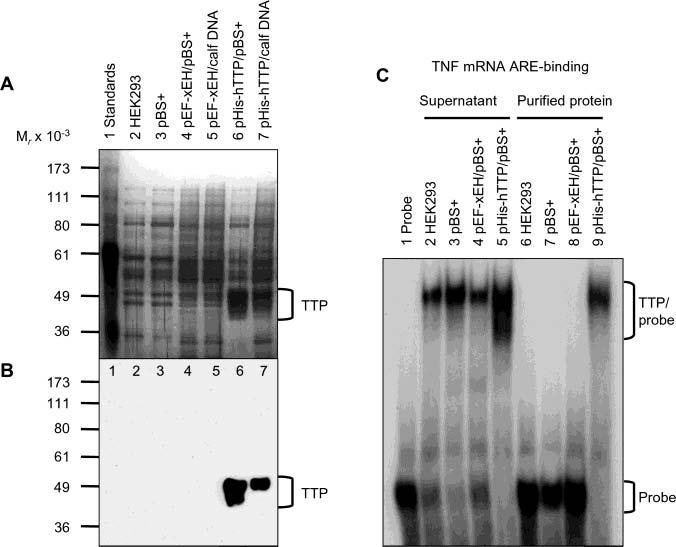
Identification of purified TTP free of endogenous ARE binding activity. HEK293 cells were transiently transfected with pHis-hTTP (0.5 μg) and cotransfected with 4.5 μg of pBS+ (lane 6) or calf thymus DNA (lane 7). As negative controls, cells were also transfected with buffers only (lane 2), pBS+ only (5 μg) (lane 3), or pEF-XEH encoding a His-tagged Xenopus EH domain of intersectin (0.5 μg) and cotransfected with 4.5 μg of pBS+ (lane 4) or calf thymus DNA (lane 5). Proteins were purified with Ni–NTA beads. (A) Silver staining. Each lane was loaded with 5 μL of the purified proteins eluted with 100 mM imidazole buffer. (B) Immunoblotting. Each lane was loaded with 5 μL of the 10000g supernatant and detected with the anti-MBP–hTTP antibodies. (C) Gel mobility shift assay. Each assay used 4 μL of the 10000g supernatant (lanes 2–5) or 4 μL of the purified proteins in 100 mM imidazole elution buffer (lanes 6–9). The positions of TTP, TTP–probe complexes, and free probes are indicated.
