Figure 5.
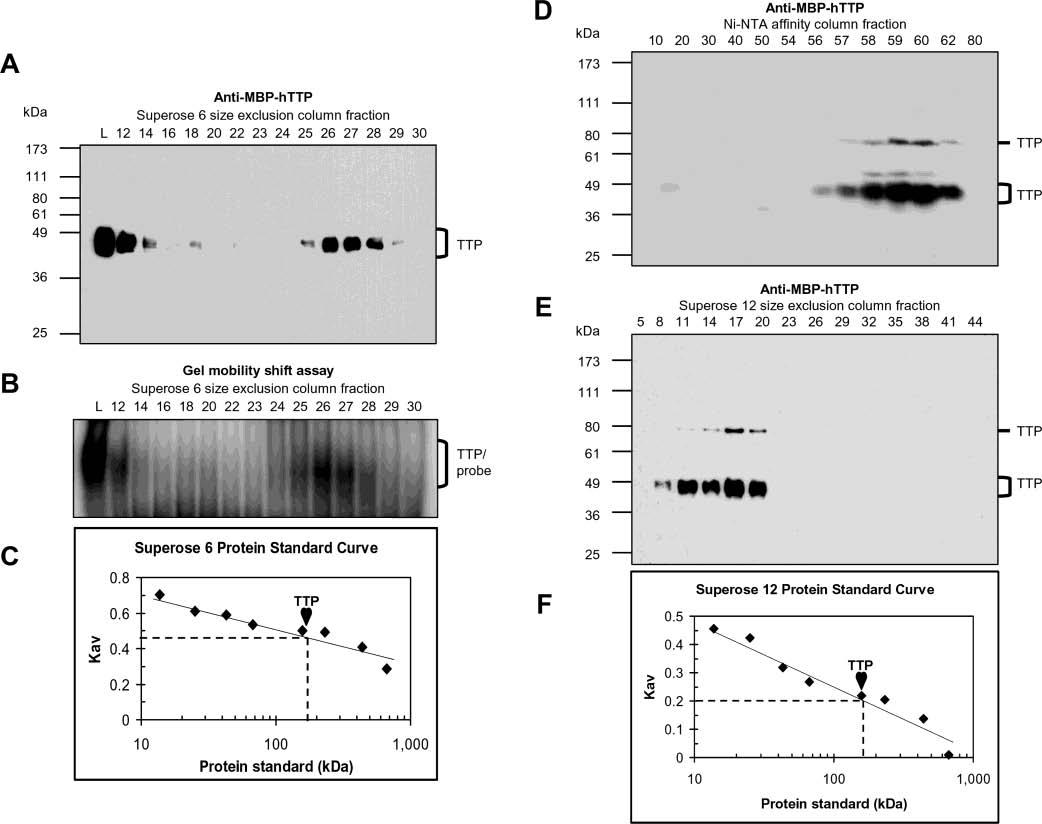
Size of active TTP from transfected human cells. The size of TTP was estimated by size exclusion chromatography using the Superose 6 column and the 10000g supernatant from HEK293 cells transfected with pHis-hTTP (A–C) and using the Superose 12 column and TTP purified by a Ni–NTA affinity column from HEK293T cells transfected with pHis-hTTP (D–F). Fractions were analyzed for the presence of TTP by immunoblotting with anti-MBP–hTTP antibodies (A, D, and E) and analyzed for ARE binging activity by GMSA (B). Lane L in panels A and B represents the initial proteins loaded in the size exclusion columns. The positions of TTP and TTP–probe complexes are indicated. The size of TTP was estimated using a standard curve generated with protein standards run on the same column under identical conditions (C and F), in which Kav = (Ve − Vo)/(Vt − Vo), where Ve, Vo, and Vt are the elution volume of the protein determined by the experiment, the void volume determined with blue dextran (2000 kDa), and the bed volume of the column provided by the manufacturer (Amersham), respectively. The protein standards that were used were provided by the manufacturer (Amersham): bovine pancreas ribonuclease A (13.7 kDa), bovine pancreas chymotrypsinogen (25 kDa), hen egg ovalbumin (43 kDa), bovine serum albumin (67 kDa), rabbit muscle aldolase (158 kDa), bovine liver catalase (232 kDa), horse spleen ferritin (440 kDa), and bovine thyroid thyroglobulin (669 kDa). Similar results were obtained using the Superose 12 column and the same extracts as in panels A–C (data not shown).
