Figure 8.
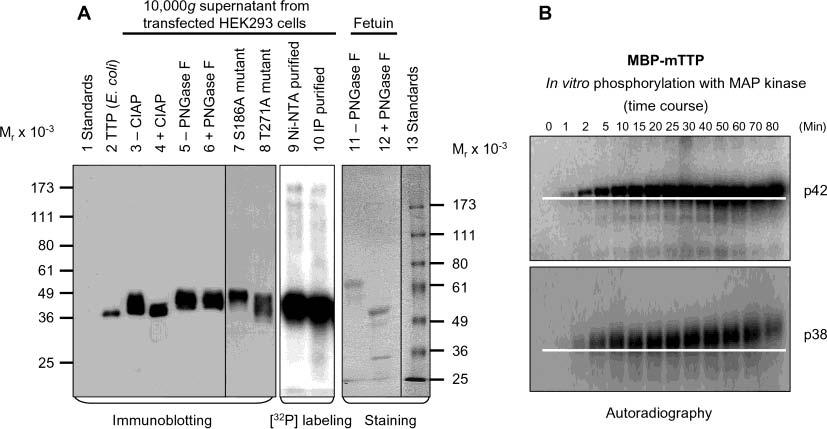
Phosphorylation of TTP in vivo and in vitro. (A) The 10000g supernatant from HEK293 cells transfected with wild-type pHish-TTP was treated with or without CIAP (lanes 3 and 4) or with or without PNGase F (lanes 5 and 6). Nonfusion hTTP purified from E. coli cells as shown in Figure 7A (lane 2) was used as a size standard for estimating the extent of the dephosphorylation (lane 2). HEK293 cells transfected with the wild-type plasmid were also labeled with [32P]orthophosphate in vivo. TTP was then purified from the 10000g supernatant of the labeled cells by affinity purification using Ni–NTA beads (lane 9) or by immunoprecipitation using the anti-MBP–hTTP serum (lane 10). The 10000g supernatants from HEK293 cells transfected with two mutant plasmids (S186A and T271A) are also shown (lanes 7 and 8). Proteins were separated by 10% SDS–PAGE, and TTP was detected with anti-MBP–hTTP antibodies (lanes 2–8). The labeled protein was detected by autoradiography (lanes 9 and 10). As a positive control for deglycosylation, fetuin was treated with or without PNGase F under identical conditions and detected with Coomassie blue staining (lanes 11 and 12). (B) Phosphorylation of MBP–mTTP by MAP kinases in vitro. MBP–mTTP (1 μM) purified from E. coli by amylose affinity resin was treated with MAP kinases, including p42/ERK2 (top) and p38 (bottom). Aliquots of the reaction mixtures were taken at various times as indicated and separated by 10% SDS–PAGE. The gels were exposed to X-ray film. A white line on the image highlights the gel mobility shift of the phosphorylated protein. Similar results were obtained with JNK, and with MBP–hTTP as the substrate (data not shown).
