Abstract
1. A study has been made, in the isolated, beating dog heart perfused with blood, of the transcapillary exchange of the following substances: [3H]water, 22Na, 86Rb, 36Cl, [14C]urea, [3H]glycerol, [3H]glucose, [14C]sucrose and [3H]inulin.
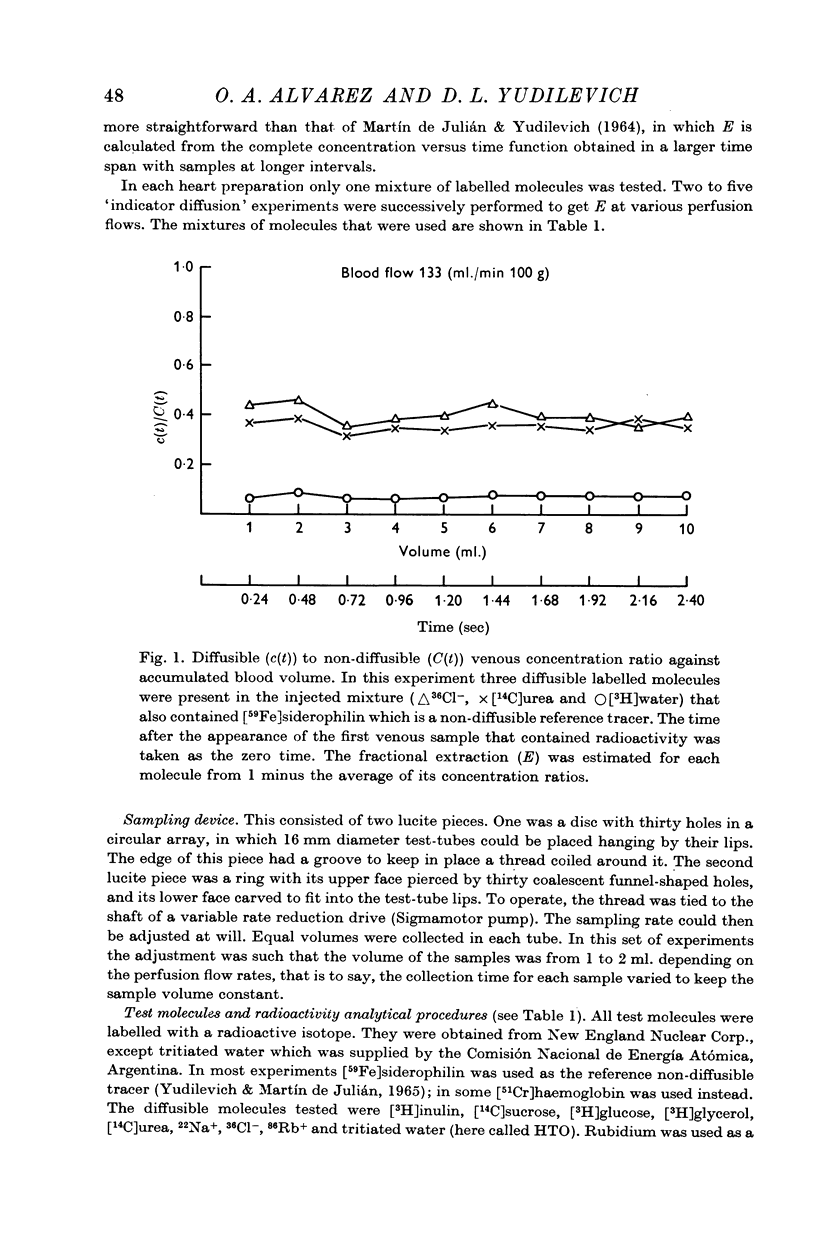
2. The method used to study the exchange was the `indicator diffusion' technique. It consists in a rapid arterial injection of a mixture containing a diffusible and a non-diffusible molecule, followed by a rapid split collection of the venous outflow, up to 30 sec. The fractional extraction, E, of the diffusible substance was obtained by comparing the relative concentrations of both tracers in injected medium and in each venous sample.
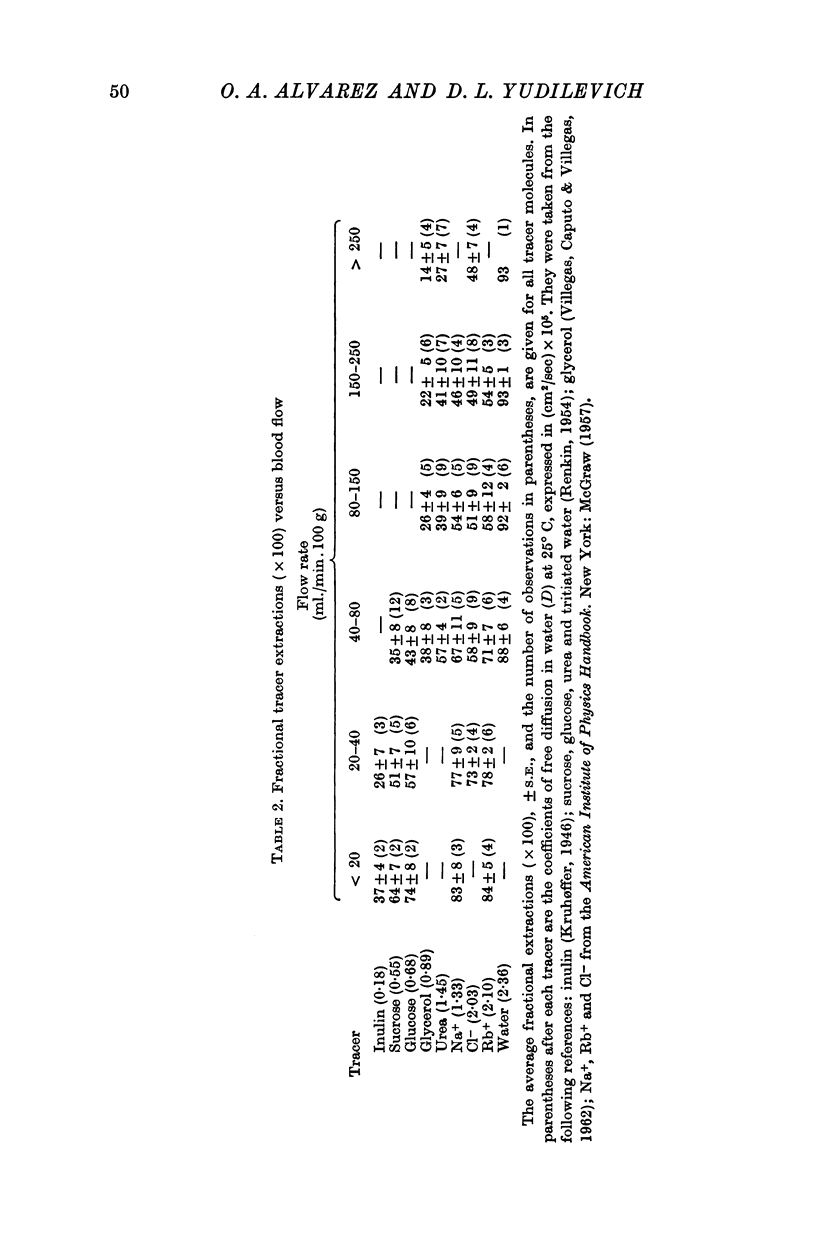
3. E for [3H]water was the highest (0·90 ± 0·3), and it did not vary with flow. All other molecules had values for E that decreased as flow increased.
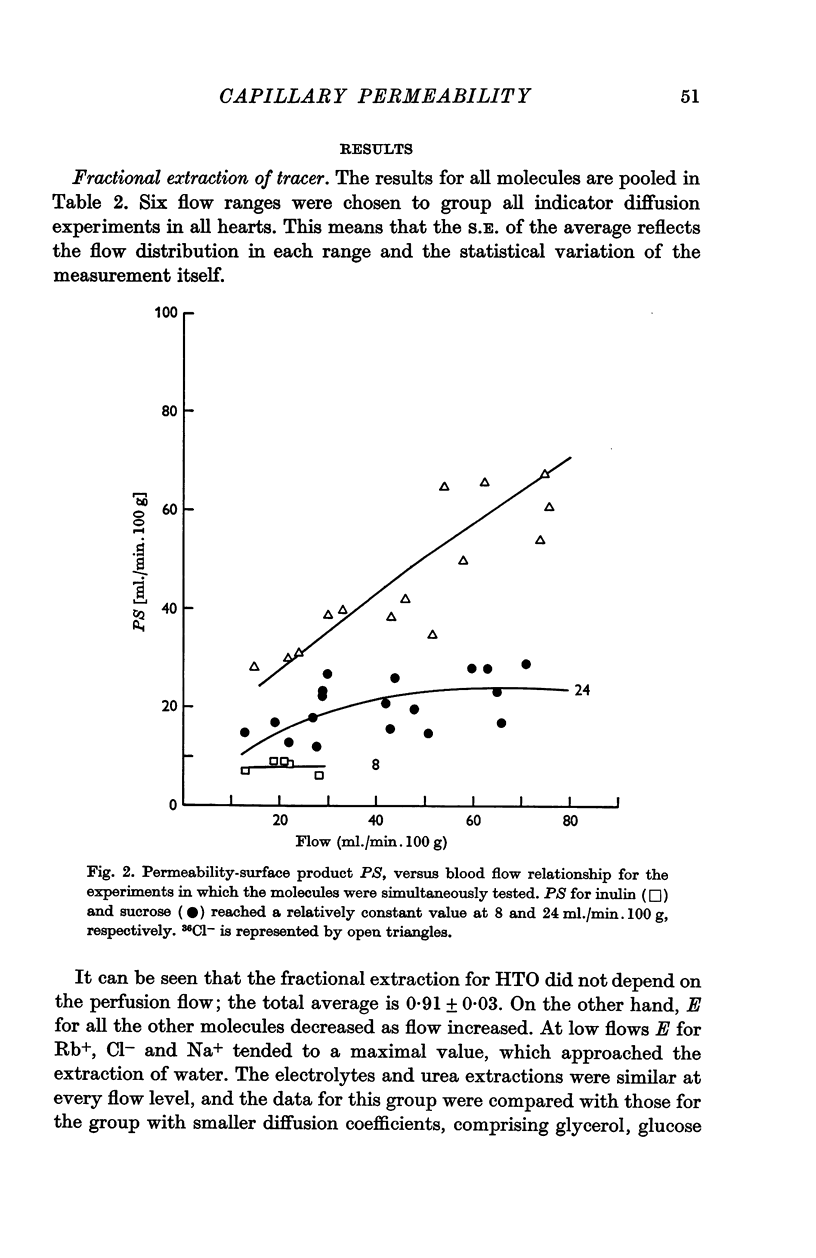
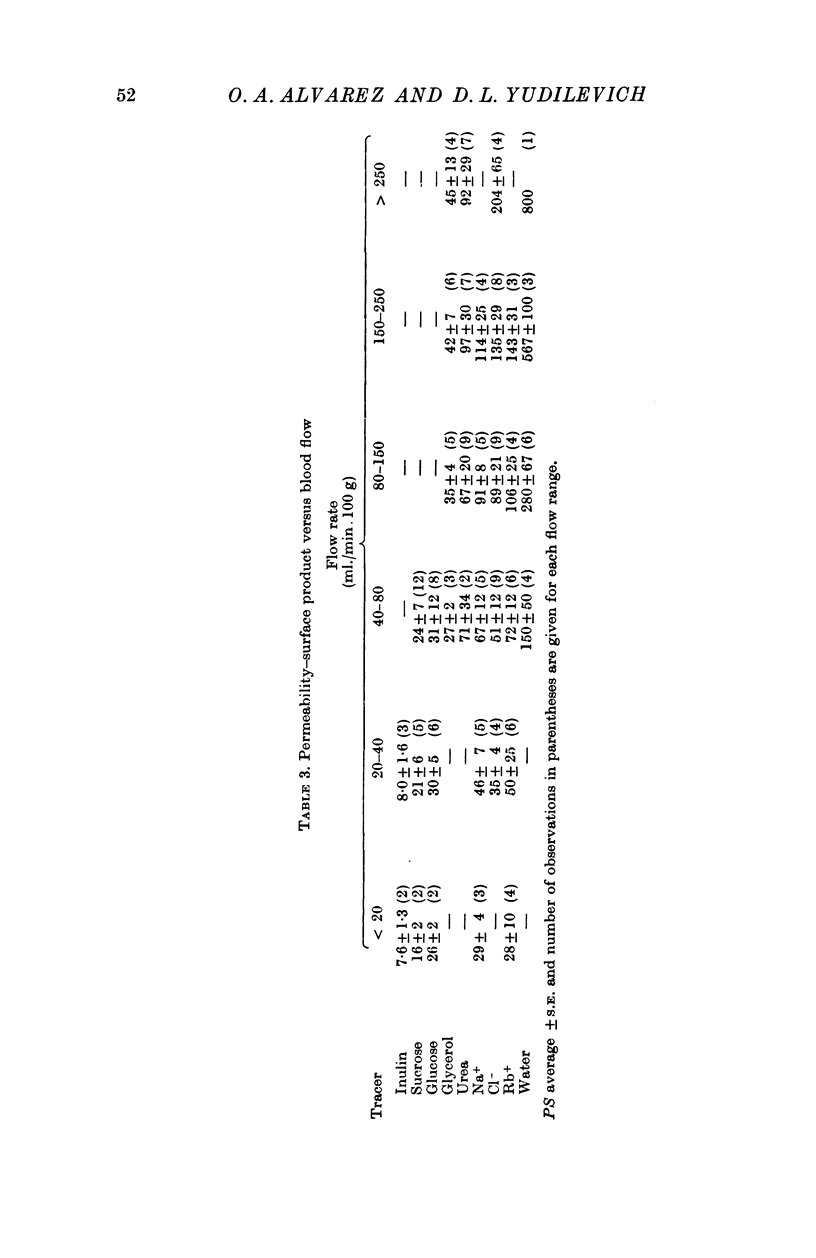
4. Capillary permeability constant, P, was estimated from PS = — F ln (1 — E), in which S is the surface area of exchange and F is the blood perfusion rate. To test the validity of the equation, E was measured at different blood perfusion rates. It was found that the equation did not apply at relatively low flows for the more diffusible substances.
5. The average values of P estimated for inulin, sucrose, glucose, glycerol and urea were 0·27, 0·8, 1·0, 1·5 and 3·1 × 105 (cm/sec), respectively. The ratio P/D (in which D is the free diffusion in water constant) was the same for all these substances. This can be interpreted as showing that if pores exist in the capillary endothelium, they must be larger than 80-100 Å diameter. It is concluded that the pores could actually be the intercellular slits as previously suggested by electron microscope studies. Endothelial cell participation in the exchange appears to be small except for [3H]water.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O. A., Yudilevich D. L. Capillary permeability and tissue exchange of water and electrolytes in the stomach. Am J Physiol. 1967 Aug;213(2):315–322. doi: 10.1152/ajplegacy.1967.213.2.315. [DOI] [PubMed] [Google Scholar]
- BENNETT H. S., LUFT J. H., HAMPTON J. C. Morphological classifications of vertebrate blood capillaries. Am J Physiol. 1959 Feb;196(2):381–390. doi: 10.1152/ajplegacy.1959.196.2.381. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T., GORESKY C. A., NOLAN M. F. RENAL TRANSIT TIMES AND DISTRIBUTION VOLUMES OF T-1824, CREATININE, AND WATER. Am J Physiol. 1965 Aug;209:243–252. doi: 10.1152/ajplegacy.1965.209.2.243. [DOI] [PubMed] [Google Scholar]
- CRONE C. Does "restricted diffusion" occur in muscle capillaries? Proc Soc Exp Biol Med. 1963 Feb;112:453–455. doi: 10.3181/00379727-112-28075. [DOI] [PubMed] [Google Scholar]
- CRONE C. THE PERMEABILITY OF CAPILLARIES IN VARIOUS ORGANS AS DETERMINED BY USE OF THE 'INDICATOR DIFFUSION' METHOD. Acta Physiol Scand. 1963 Aug;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Crone C. The permeability of brain capillaries to non-electrolytes. Acta Physiol Scand. 1965 Aug;64(4):407–417. doi: 10.1111/j.1748-1716.1965.tb04198.x. [DOI] [PubMed] [Google Scholar]
- GRIM E. Relation between pressure and concentration difference across membranes permeable to solute and solvent. Proc Soc Exp Biol Med. 1953 Jun;83(2):195–200. doi: 10.3181/00379727-83-20306. [DOI] [PubMed] [Google Scholar]
- Hanai T., Haydon D. A., Redwood W. R. The water permeability of artificial bimolecular leaflets: a comparison of radio-tracer and osmotic methods. Ann N Y Acad Sci. 1966 Jul 14;137(2):731–739. doi: 10.1111/j.1749-6632.1966.tb50194.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Wilson T. A. A model for capillary exchange. Am J Physiol. 1966 Jun;210(6):1299–1303. doi: 10.1152/ajplegacy.1966.210.6.1299. [DOI] [PubMed] [Google Scholar]
- KAHN J. B., Jr The entry of rubidium into human erythrocytes. J Pharmacol Exp Ther. 1962 May;136:197–204. [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- MARTIN P., YUDILEVICH D. A THEORY FOR THE QUANTIFICATION OF TRANSCAPILLARY EXCHANGE BY TRACER-DILUTION CURVES. Am J Physiol. 1964 Jul;207:162–168. doi: 10.1152/ajplegacy.1964.207.1.162. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., RENKIN E. M., BORRERO L. M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951 Oct;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol. 1959 Dec;197:1205–1210. doi: 10.1152/ajplegacy.1959.197.6.1205. [DOI] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFER D. E., JOHNSON J. A. PERMEABILITY OF MAMMALIAN HEART CAPILLARIES TO SUCROSE AND INULIN. Am J Physiol. 1964 May;206:985–991. doi: 10.1152/ajplegacy.1964.206.5.985. [DOI] [PubMed] [Google Scholar]
- STUBBS J., WIDDAS W. F. Extravascular fluid losses in the perfused isolated rabbit heart. J Physiol. 1959 Oct;148:393–402. doi: 10.1113/jphysiol.1959.sp006295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland T. M., Young D. A. Increased permeability of the capillaries of the rat heart to plasma albumin with asphyxiation and with perfusion. J Physiol. 1966 Mar;183(1):112–122. doi: 10.1113/jphysiol.1966.sp007854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H. Transport through biological membranes. Annu Rev Physiol. 1953;15:1–20. doi: 10.1146/annurev.ph.15.030153.000245. [DOI] [PubMed] [Google Scholar]
- VARGAS F., JOHNSON J. A. AN ESTIMATE OF REFLECTION COEFFICIENTS FOR RABBIT HEART CAPILLARIES. J Gen Physiol. 1964 Mar;47:667–677. doi: 10.1085/jgp.47.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLEGAS R., CAPUTO C., VILLEGAS L. Diffusion barrieres in the squid nerve fiber. The axolemma and the Schwann layer. J Gen Physiol. 1962 Nov;46:245–255. doi: 10.1085/jgp.46.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas F., Johnson J. A. Permeability of rabbit heart capillaries to nonelectrolytes. Am J Physiol. 1967 Jul;213(1):87–93. doi: 10.1152/ajplegacy.1967.213.1.87. [DOI] [PubMed] [Google Scholar]
- WOLF A. V., REMP D. G., KILEY J. E., CURRIE G. D. Artificial kidney function; kinetics of hemodialysis. J Clin Invest. 1951 Oct;30(10):1062–1070. doi: 10.1172/JCI102526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUDILEVICH D., MARTINDEJULIAN P. POTASSIUM, SODIUM, AND IODIDE TRANSCAPILLARY EXCHANGE IN THE DOG HEART. Am J Physiol. 1965 May;208:959–967. doi: 10.1152/ajplegacy.1965.208.5.959. [DOI] [PubMed] [Google Scholar]
- Young D. A. Factors controlling the washout of the interstitial space of the isolated, perfused rat heart. J Physiol. 1968 Jun;196(3):747–759. doi: 10.1113/jphysiol.1968.sp008534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudilevich D. L., Alvarez O. A. Water, sodium, and thiourea transcapillary diffusion in the dog heart. Am J Physiol. 1967 Aug;213(2):308–314. doi: 10.1152/ajplegacy.1967.213.2.308. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Renkin E. M., Alvarez O. A., Bravo I. Fractional extraction and transcapillary exchange during continuous and instantaneous tracer administration. Circ Res. 1968 Aug;23(2):325–336. doi: 10.1161/01.res.23.2.325. [DOI] [PubMed] [Google Scholar]


